Description
Trifluoromethanesulfonic acid, reagent grade, 98%, 50g
Synonym(s):
TFMSA, Triflic acid
Linear Formula:
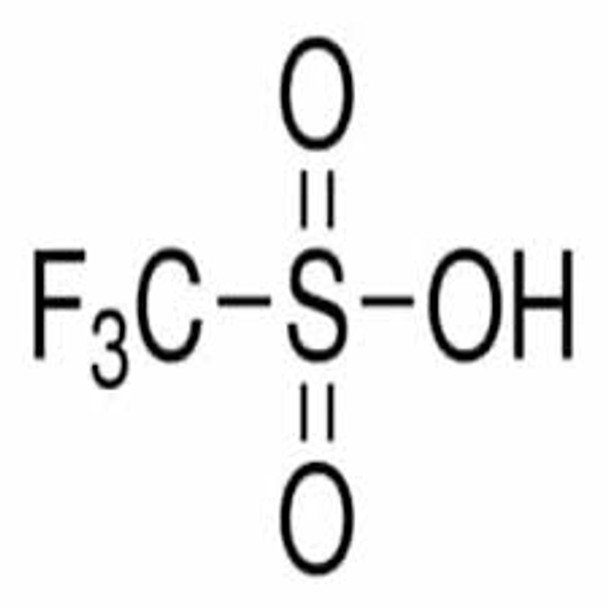
CF3SO3H
CAS Number:
1493-13-6
Molecular Weight:
150.08
Beilstein:
1812100
EC Number:
216-087-5
MDL number:
MFCD00007514
PubChem Substance ID:
24849764
NACRES:
NA.21
PROPERTIES
grade
reagent grade
Quality Level
200
vapor density
5.2 (vs air)
vapor pressure
8 mmHg ( 25 °C)
assay
98%
form
liquid
refractive index
n20/D 1.327 (lit.)
bp
162 °C (lit.)
density
1.696 g/mL at 25 °C (lit.)
SMILES string
OS(=O)(=O)C(F)(F)F
InChI
1S/CHF3O3S/c2-1(3,4)8(5,6)7/h(H,5,6,7)
InChI key
ITMCEJHCFYSIIV-UHFFFAOYSA-N
General description
Trifluoromethanesulfonic acid is the strongest monoprotic organic acid. It has been synthesized by the oxidation of bis(trifluoromethylthio)mercury with aqueous hydrogen peroxide.[1] It undergoes complete dissociation in basic solvents such as dimethyl sulfoxide, dimethylacetamide and dimethylformamide. Its dissociation in non-aqueous solvents has been studied by conductometry.[1] On mixing trifluoromethanesulfonic acid with HNO3, it forms nitronium trifluoromethane sulfonate, which is an excellent nitrating reagent.[2]
Application
Trifluoromethanesulfonic acid is a versatile reagent[3], employed as catalyst for the following studies:
- Friedel-Crafts acylation of aromatic compounds with methyl benzoate.[4]
- Addition reaction of dialkyl disulfides to terminal alkynes.[5]
- Synthesis of a single cyclic tetrasiloxane containing propylammonium trifluoromethanesulfonate and methyl side-chain groups (Am-CyTS).[6]
- Preparation of starting reagents for the synthesis of fluorinated 2,5-substituted 1-ethyl-1H-benzimidazole derivatives.[7]
- Synthesis of aryl triflates,[8] the lactonization of alkenoic acids,[9] and the formation of E-alkenes.[10]
Trifluoromethanesulfonic acid may be used as an initiator for the cationic polymerization of styrene[11], hexamethylcyclotrisiloxane[12] and L,L-dilactide.[13]
Deglycosylation agent
Packaging
10, 50, 100 g in ampule
SAFETY INFORMATION
Pictograms
GHS05,GHS07
Signal Word
Danger
Hazard Statements
H290 - H302 - H314 - H335
Precautionary Statements
P234 - P261 - P280 - P301 + P312 - P303 + P361 + P353 - P305 + P351 + P338
Hazard Classifications
Acute Tox. 4 Oral - Met. Corr. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible, corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
332.1 °F - Pensky-Martens closed cup
Flash Point(C)
> 166.7 °C - Pensky-Martens closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves