Description
Trifluoroacetic Acid, 1L
ReagentPlus®, 99%
Synonym(s):
TFA
Linear Formula:
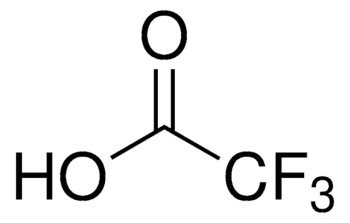
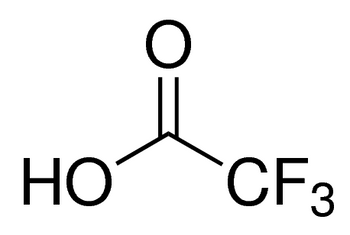
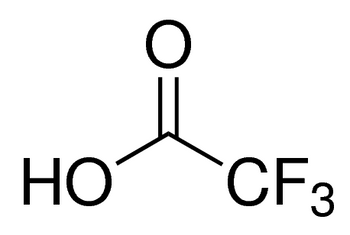
CF3COOH
CAS Number:
76-05-1
Molecular Weight:
114.02
Beilstein:
742035
EC Number:
200-929-3
MDL number:
MFCD00004169
PubChem Substance ID:
329826846
NACRES:
NA.21
PROPERTIES
vapor density
3.9 (vs air)
Quality Level
200
vapor pressure
97.5 mmHg ( 20 °C)
product line
ReagentPlus®
Assay
99%
form
liquid
impurities
≤0.05% water
refractive index
n20/D 1.3 (lit.)
pH
1 (10 g/L)
bp
72.4 °C (lit.)
mp
−15.4 °C (lit.)
solubility
ethanol: soluble 0.33 mL/mL
density
1.489 g/mL at 20 °C (lit.)
SMILES string
OC(C(F)(F)F)=O
InChI
1S/C2HF3O2/c3-2(4,5)1(6)7/h(H,6,7)
InChI key
DTQVDTLACAAQTR-UHFFFAOYSA-N
General description
Trifluoroacetic acid (TFA) is an organofluorine compound used as a reagent in organic synthesis for various acid-catalyzed reactions such as ring-opening of epoxides, biomimetic cyclization, Cope rearrangements, and natural product synthesis. TFA′s physicochemical characteristics provide advantages over other acids because of its high volatility, solubility in organic solvents, and acidic strength. When TFA is used as a reagent the product isolation is simple by evaporation due to its very high volatility. Less volatile acids such as sulfuric acid or p-toluenesulfonic acid may require neutralization or an extractive workup.[1]
Application
Trifluoroacetic acid can be used as a reagent:
TFA can also be used as:
- For the cleavage of nitrogen and oxygen protecting groups such as N-Boc, N-benzyloxymethyl, benzyl ether, p-methoxybenzyl ether, t-butyl ether, t-butyloxymethyl ether, triphenylmethyl ether, and dimethyl acetals.[1][2][3]
- In the Baeyer–Villiger oxidation reactions in combination with sodium percarbonate.[1],·
- For the C-H trifluoromethylation of arenes.[4]
TFA can also be used as:
- A solvent in atom transfer cyclization reactions and polymer processes.[2]
- A catalyst in the synthesis of ε-caprolactam via Beckmann rearrangement of cyclohexanone oxime in aprotic solvents.[5]
Packaging
1mL in each ampule.
Legal Information
ReagentPlus is a registered trademark of Sigma-Aldrich Co. LLC