Description
Toluene ACS reagent, ≥99.5%, 4L
-
CAS Number 108-88-3
-
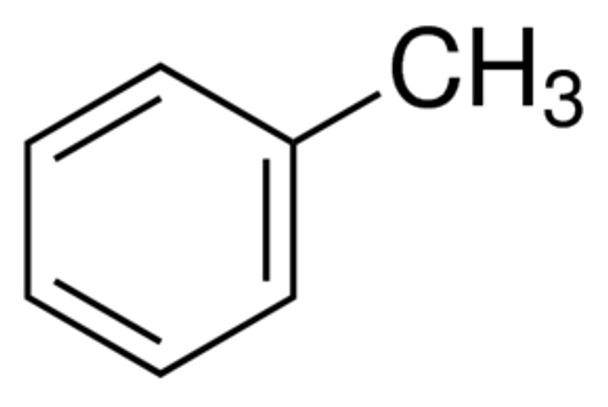
Linear Formula C6H5CH3
-
Molecular Weight 92.14
-
Beilstein/REAXYS Number 635760
-
EC Number 203-625-9
-
MDL number MFCD00008512
-
eCl@ss 39011102
-
PubChem Substance ID 329751592
-
NACRES NA.21
Properties
| Related Categories | ACS Grade, ACS Grade Solvents, ACS and Reagent Grade Solvents, Amber Glass Bottles, Carbon Steel Cans with NPT Threads, Core Bioreagents, Life Science Reagents for Transfection, Research Essentials, Semi-Bulk Solvents, Solvent by Type, Solvent Bottles, Solvent Packaging Options, Solvent by Application, Solvents, Toluene |
| Quality Level | 200 |
| grade | ACS reagent |
| vapor density | 3.2 (vs air) |
| vapor pressure | 22 mmHg ( 20 °C) |
| - | 26 mmHg ( 25 °C) |
| assay | ≥99.5% |
| form | liquid |
| autoignition temp. | 997 °F |
| expl. lim. | 7 % |
| application(s) | microbiology: suitable |
| impurities | H2SO4, passes test (darkened) |
| - | ≤0.003% S compounds |
| - | ≤0.030% water |
| evapn. residue | ≤0.0010% |
| color | APHA: ≤10 |
| refractive index | n/D 1.496 (lit.) |
| bp | 110-111 °C (lit.) |
| mp | -93 °C (lit.) |
| density | 0.865 g/mL at 25 °C (lit.) |
| SMILES string | Cc1ccccc1 |
| InChI | 1S/C7H8/c1-7-5-3-2-4-6-7/h2-6H,1H3 |
| InChI key | YXFVVABEGXRONW-UHFFFAOYSA-N |
General description
Toluene, a flammable liquid with a pungent odor, is widely employed as organic solvent. It is widely used as a precursor for synthesizing benzene and as a solvent in the paint industry.[6] It has been reported to be a biotoxic solvent (toxic to many microorganisms at 0.1%v/v concentrations).[1] Its anaerobic biodegradation to CO2, by the denitrifying bacterium Thauera arornatica has been reported.[2] It forms a syndiotactic polystyrene-toluene molecular compound. Crystal structure of this molecular compound has been investigated by X-ray diffraction studies.[3]
Application
Toluene has been employed as an solvent for the asymmetric synthesis of propargylic alcohols via addition reaction of terminal alkynes with various aldehydes in the presence of an optically active reagent, N-methylephedrine.[4] It may be used in the preparation of amine-capped gold nanocrystals.[5] Toluene undergoes alkylation in the presence of modified ZSM (Zeolite Socony Mobil)-5-class zeolite catalysts to form p-xylene with high selectivity.[8] When doped on graphene, it acts as an electron donor leading to alteration in graphene electrical properties.[7]
Packaging
1, 6×1, 2.5, 4×2.5, 4, 4×4 L in glass bottle
View returnable container options.
18, 20, 200 L in steel drum
500, 6×500 mL in glass bottle