MilliporeSigma
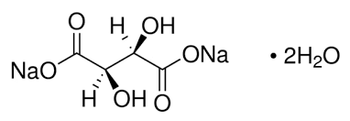
Sodium hyaluronate for IR Identification, United States Pharmacopeia (USP) Reference Standard, 250MG
- SKU:
- 1614159-250MG
Description
Sodium hyaluronate for IR Identification, United States Pharmacopeia (USP) Reference Standard, 250MG
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
Hyaluronic acid sodium salt
CAS Number:
9067-32-7
NACRES:
NA.24
PROPERTIES
grade
pharmaceutical primary standard
manufacturer/tradename
USP
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
−20°C
InChI
1S/C28H44N2O23.Na/c1-5(33)29-9-18(11(35)7(3-31)47-25(9)46)49-28-17(41)15(39)20(22(53-28)24(44)45)51-26-10(30-6(2)34)19(12(36)8(4-32)48-26)50-27-16(40)13(37)14(38)21(52-27)23(42)43;/h7-22,25-28,31-32,35-41,46H,3-4H2,1-2H3,(H,29,33)(H,30,34)(H,42,43)(H,44,45);/q;+1/t7-,8-,9-,10-,11-,12-,13+,14+,15-,16-,17-,18-,19-,20+,21+,22+,25-,26+,27-,28-;/m1./s1
InChI key
YWIVKILSMZOHHF-QJZPQSOGSA-N
DESCRIPTION
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including MSDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.
Analysis Note
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
SAFETY INFORMATION
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable