Description
N,N-Dimethylformamide, 2L
Synonym(s):
DMF, NSC 5356
Linear Formula:
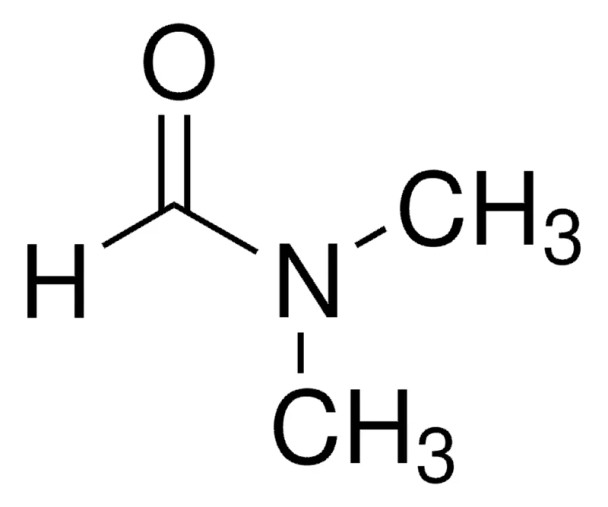
HCON(CH3)2
CAS Number:
68-12-2
Molecular Weight:
73.09
Beilstein:
605365
EC Number:
200-679-5
MDL number:
MFCD00003284
PubChem Substance ID:
57647999
NACRES:
NA.21
PROPERTIES
grade
anhydrous
Quality Level
300
vapor density
2.5 (vs air)
vapor pressure
2.7 mmHg ( 20 °C)
Assay
99.8%
form
liquid
autoignition temp.
833 °F
expl. lim.
15.2 %
impurities
<0.005% water
evapn. residue
<0.0005%
refractive index
n20/D 1.430 (lit.)
pH
7 (20 °C, 200 g/L)
bp
153 °C (lit.)
mp
−61 °C (lit.)
density
0.944 g/mL (lit.)
SMILES string
[H]C(=O)N(C)C
InChI
1S/C3H7NO/c1-4(2)3-5/h3H,1-2H3
InChI key
ZMXDDKWLCZADIW-UHFFFAOYSA-N
General description
N,N-Dimethylformamide (DMF) is the commonly employed solvent for chemical reactions. DMF is a useful solvent employed for the isolation of chlorophyll from plant tissues.[1] It is widely employed reagent in organic synthesis. It plays multiple roles in various reactions such as solvent, dehydrating agent, reducing agent as well as catalyst. It is a multipurpose building block for the synthesis of compounds containing O, -CO, -NMe2, -CONMe2, -Me, -CHO as functional groups.[2]
N,N-Dimethylformamide is a polar solvent commonly used in organic synthesis. It also acts as a multipurpose precursor for formylation, amination, aminocarbonylation, amidation and cyanation reactions.[2]
Application
N,N-Dimethylformamide (anhydrous) has been used as solvent for the synthesis of cytotoxic luteinizing hormone-releasing hormone (LH-RH) conjugate AN-152 (a chemotherapeutic drug) and fluorophore C625 [4-(N,N-diphenylamino)-4′-(6-O-hemiglutarate)hexylsulfinyl stilbene].[3] It may be employed as solvent medium for the various organic reduction reactions.[4]
DMF has been used as a solvent in the following processes:
- Multi-step synthesis of L-azidohomoalanine (L-Aha) during the substitution of the mesylate by sodium azide.[5]
- Synthesis of phosphine-FLAG®, a detection reagent for metabolic labeling of glycans.[6]
- Synthesis of per-O-acetylated 6-azidofucose, a per-O-acetylated azido sugar.[6]
Solvent for many hydrophobic organic compounds.
Legal Information
FLAG is a registered trademark of Merck KGaA, Darmstadt, Germany
SAFETY INFORMATION
Pictograms
GHS02,GHS07,GHS08
Signal Word
Danger
Hazard Statements
H226 - H312 + H332 - H319 - H360D
Precautionary Statements
P210 - P280 - P303 + P361 + P353 - P304 + P340 + P312 - P305 + P351 + P338 - P308 + P313
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Eye Irrit. 2 - Flam. Liq. 3 - Repr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
135.5 °F - closed cup
Flash Point(C)
57.5 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves