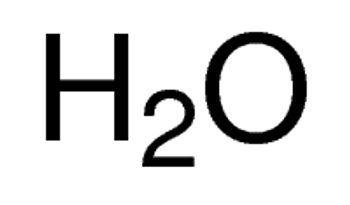Description
Product Details:
-
CAS Number 67-56-1
-
Linear Formula CH3OH
-
Molecular Weight 32.04
-
Beilstein Registry Number 1098229
-
EC Number 200-659-6
-
MDL number MFCD00004595
- Specification Sheet
- SDS
Properties:
| grade | for HPLC |
| vapor density | 1.11 (vs air) |
| vapor pressure | 410 mmHg ( 50 °C) |
| 97.68 mmHg ( 20 °C) | |
| assay | ≥99.9% |
| autoignition temp. | 725 °F |
| expl. lim. | 36 % |
| impurities | ≤0.0005% non-volatile matter |
| ≤1 ppb fluorescence (quinine) at 254 nm | |
| ≤1 ppb fluorescence (quinine) at 365 nm | |
| <0.03% water (Karl Fischer) | |
| evapn. residue | <0.0005% |
| color | APHA: ≤10 |
| refractive index | n20/D 1.329(lit.) |
| bp | 64.7 °C(lit.) |
| mp | −98 °C(lit.) |
| density | 0.791 g/mL at 25 °C(lit.) |
| λ | neat |
| UV absorption | λ: 205 nm Amax: ≤1.00 |
| λ: 210 nm Amax: ≤0.60 | |
| λ: 220 nm Amax: ≤0.30 | |
| λ: 230 nm Amax: ≤0.20 | |
| λ: 235 nm Amax: ≤0.10 | |
| λ: 240 nm Amax: ≤0.10 | |
| λ: 260 nm Amax: ≤0.04 | |
| λ: 280 nm Amax: ≤0.01 | |
| λ: 400 nm Amax: ≤0.01 |
Packaging
4×4 L in glass bottle
Preparation Note
Product filtered through a 0.2 μm filter
Application
Methanol (MeOH) has been used in the wholemount immunofluorescence studies of embryonic tissues.[7]
It may be used in the following studies:
• Colony forming unit-fibroblast assay of bone marrow mononuclear cells.[1]
• As solvent for the preparation of extracts of hyphae of Aspergillus for the estimation of gliotoxin by reversed phase-HPLC.[4]
• Immunofluorescence studies.[5]
• To compose the eluent for the ion-pair reverse-phase HPLC isolation of nucleotides and their decomposition products.[6]
Methanol can be used as a mobile phase in reversed-phase liquid chromatography.[12]
General description
Methanol is an organic solvent that can be synthesized from syngas in the presence of CuO/ZnO/Al2O3 catalysts.[8] It is an ideal candidate as a hydrogen source in fuel cell technology due to its high H/C ratio, low propensity for soot generation, relatively low reforming temperature and as it exists in liquid state at room temperature.[9][10] In a direct methanol fuel cell (DMFC), methanol undergoes oxidation with air to generate electricity. The olefins (ethylene or propylene) formed from methanol via MTO (methanol-to-olefins) process, can be an alternate to oil and gas to produce hydrocarbon fuels.[11]
Thermochemical conversion of methanol to C2-C10 hydrocarbons in the presence of shape-selective zeolites has been reported.[2] Its oxidation on Ru-Pt catalyst system by ruthenium ad-atoms has been proposed.[3]
Recommended products
Discover LiChropur reagents ideal for HPLC or LC-MS analysis
Legal Information
Pure-Pac is a registered trademark of Sigma-Aldrich Co. LLC