MilliporeSigma
Melatonin, Pharmaceutical Secondary Standard; Certified Reference Material, 500MG
- SKU:
- PHR1767-500MG
Description
Melatonin, Pharmaceutical Secondary Standard; Certified Reference Material, 500MG
Synonym(s):
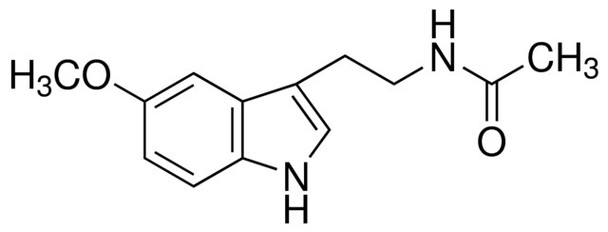
Melatonin, N-Acetyl-5-methoxytryptamine
Empirical Formula (Hill Notation):
C13H16N2O2
CAS Number:
73-31-4
Molecular Weight:
232.28
Beilstein:
205542
EC Number:
200-797-7
MDL number:
MFCD00005655
PubChem Substance ID:
329823599
NACRES:
NA.24
PROPERTIES
grade
certified reference material
pharmaceutical secondary standard
Quality Level
300
Agency
traceable to BP 1077
traceable to USP 1380105
form
solid
CofA
current certificate can be downloaded
packaging
pkg of 500 mg
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
mp
116.5-118 °C (lit.)
application(s)
pharmaceutical (small molecule)
storage temp.
2-8°C
SMILES string
COc1ccc2[nH]cc(CCNC(C)=O)c2c1
InChI
1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16)
InChI key
DRLFMBDRBRZALE-UHFFFAOYSA-N
General description
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. It is analyzed using GMP validated instruments as per pharmacopeia monograph methods and is traceable to Unites States Pharmacopeia (USP), European Pharmacopeia (EP), and British Pharmacopeia (BP) primary standards, wherever applicable. It is provided with a comprehensive certificate of analysis (CoA) containing a certified purity value, calculated by the mass balance approach.
All information regarding the use of this CRM can be found on the certificate of analysis.
Melatonin is a methoxyindole compound secreted by the pineal gland in the mammalian brain. It regulates the sleep-wake cycles (circadian rhythm) and also has anti-inflammatory and antioxidant properties.
All information regarding the use of this CRM can be found on the certificate of analysis.
Melatonin is a methoxyindole compound secreted by the pineal gland in the mammalian brain. It regulates the sleep-wake cycles (circadian rhythm) and also has anti-inflammatory and antioxidant properties.
Application
Melatonin pharmaceutical reference standard may also be used as follows:
- Development and validation of a high-performance liquid chromatography (HPLC) method combined with UV detection to determine melatonin in capsule dosage forms
- Determination of melatonin in dog plasma samples by liquid chromatography-tandem mass spectrometry (LC-MS/MS) method
- Simultaneous electrochemical analysis of melatonin and 5-hydroxytryptophan by poly-(melamine)/poly-(o-aminophenol) modified screen printed electrodes (SPCs) in human urine samples
- Determination of melatonin in pharmaceutical formulations by voltammetric and amperometric methods using carbon black modified glassy carbon electrode
- Electroanalytical determination of melatonin in tablet and human urine samples using a boron-doped diamond electrode and square-wave voltammetry (SWV)
- Development of nanopalladium polymeric nanocomposite modified glassy carbon electrode (GCE) for qualitative and quantitative analyses of melatonin in tablets and human urine samples by square-wave voltammetry
- Liquid chromatography-diode array detection (LC-DAD) of melatonin dose in supplements and also detection of impurities present in supplements by ultra-high-performance liquid chromatography-orbitrap mass spectrometry (UHPLC-orbitrap-MS)
Other Notes
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Footnote
To see an example of a Certificate of Analysis for this material enter LRAA8251 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
SAFETY INFORMATION
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable