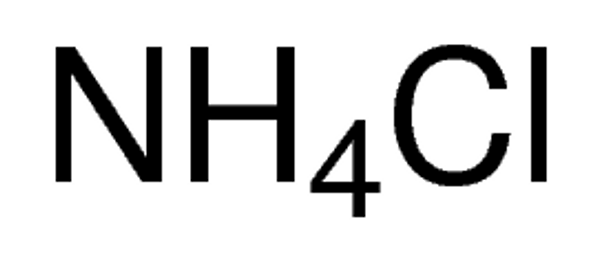Description
Ammonium chloride, ACS reagent, ≥99.5%, 500g
Synonym(s):
Salmiac
Linear Formula:
NH4Cl
CAS Number:
12125-02-9
Molecular Weight:
53.49
Beilstein/REAXYS Number:
4371014
EC Number:
235-186-4
MDL number:
MFCD00011420
eCl@ss:
38050207
PubChem Substance ID:
329752144
NACRES:
NA.21
General description
Crystals of ammonium chloride (NH4Cl) belong to the cubic crystal system. It can be synthesized by reacting HCl with aqueous ammonia solutions. NH4Cl is formed as a by-product during soda manufactured by Solvay process.
Application
Ammonium chloride (NH4Cl) has been used as one of the components of red blood cell (RBC) lysis buffer for use in various studies. It may be used as a catalyst in the preparation 3,4-dihydropyrimidin-2-(1H)-ones.
Inorganic ammonium ions (NH4+) effectively catalyze the aldol condensation of carbonyl compounds. It serves as a source of nitrogen during the preparation of trimethylamine hydrochloride (TMA·HCl).
Inorganic ammonium ions (NH4+) effectively catalyze the aldol condensation of carbonyl compounds. It serves as a source of nitrogen during the preparation of trimethylamine hydrochloride (TMA·HCl).
PROPERTIES
Quality Level
200
grade
ACS reagent
vapor density
1.9 (vs air)
vapor pressure
1 mmHg ( 160.4 °C)
assay
≥99.5%
form
powder, crystals or chunks
impurities
≤0.005% insolubles
ign. residue
≤0.01%
pH
4.5-5.5 (25 °C, 5%)
mp
340 °C (subl.) (lit.)
anion traces
phosphate (PO43-): ≤2 ppm
sulfate (SO42-): ≤0.002%
cation traces
Ca: ≤0.001%
Fe: ≤2 ppm
Mg: ≤5 ppm
heavy metals: ≤5 ppm (by ICP)