Description
Acetonitrile, suitable for HPLC, gradient grade, ≥99.9%, 18L
Synonym(s):
ACN, Cyanomethane, Ethyl nitrile, Methyl cyanide
Linear Formula:
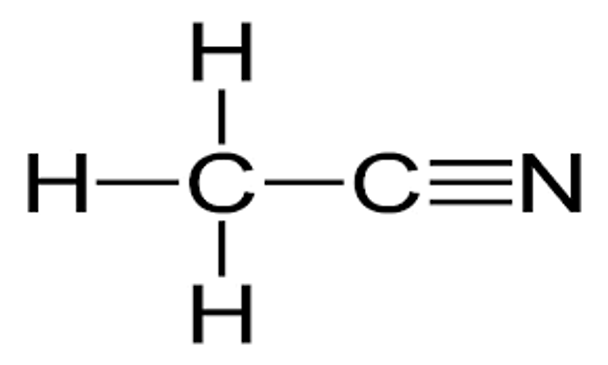
CH3CN
CAS Number:
75-05-8
Molecular Weight:
41.05
Beilstein:
741857
EC Number:
200-835-2
MDL number:
MFCD00001878
PubChem Substance ID:
329756422
NACRES:
NA.03
PROPERTIES
grade
gradient grade
Quality Level
100
vapor density
1.41 (vs air)
vapor pressure
72.8 mmHg ( 20 °C)
description
HPLC gradient analysis <= 0.5 mAU @254 nm
HPLC gradient analysis <= 3 mAU @ 210 nm
baseline drift at 210 nm <= 15 mAU
Assay
≥99.9%
form
liquid
autoignition temp.
973 °F
purified by
sub-micron filtration
expl. lim.
16 %
technique(s)
HPLC: suitable
UV/Vis spectroscopy: suitable
impurities
≤0.0002% free alkali (as NH3)
≤0.001% free acid (as CH3COOH)
≤0.01% water (Karl Fischer)
≤0.5 ppb quinine fluor., 365 nm
≤1 ppb quinine fluor., 254 nm
evapn. residue
<0.0002%
refractive index
n20/D 1.344 (lit.)
bp
81-82 °C (lit.)
mp
−45 °C (lit.)
solubility
water: soluble
density
0.786 g/mL at 25 °C (lit.)
λ
H2O reference
UV absorption
λ: 195 nm Amax: ≤0.12
λ: 200 nm Amax: ≤0.032
λ: 230 nm Amax: ≤0.0044
application(s)
food and beverages
format
neat
SMILES string
CC#N
InChI
1S/C2H3N/c1-2-3/h1H3
InChI key
WEVYAHXRMPXWCK-UHFFFAOYSA-N
Acetonitrile (MeCN), an aliphatic nitrile, is a colorless liquid with a pleasant odor. It is widely used as a solvent and intermediate in organic syntheses.[1] It is transparent to UV-visible light making it highly applicable in spectrophotometric and fluorimetric techniques. As MeCN has low viscosity, high elution strength and is highly miscible in water, it is utilized as a mobile phase component in many chromatographic techniques.[2] Its infrared spectrum has been recorded.[3] Synthesis of alkyl hydroperoxides in MeCN by alkane oxidation with hydrogen peroxide in the presence of iron complexes has been studied.[4] The hydrogenation of MeCN to form ethylamine using Co–B amorphous alloy catalyst has been investigated.[5]
Preparation Note
Product filtered through a 0.2 μm filter