Description
Acetone ACS reagent, ≥99.5%
-
CAS Number 67-64-1
-
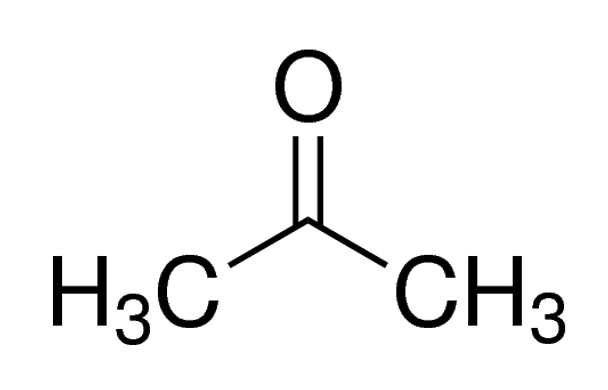
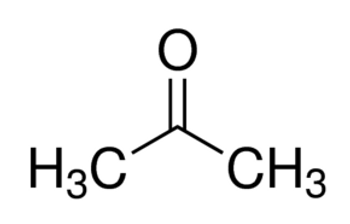
Linear Formula CH3COCH3
-
Molecular Weight 58.08
-
Beilstein/REAXYS Number 635680
-
EC Number 200-662-2
-
MDL number MFCD00008765
-
eCl@ss 39021201
-
PubChem Substance ID 329751579

-
NACRES NA.02
Properties
| Related Categories | ACS Grade, ACS Grade Solvents, ACS and Reagent Grade Solvents, Acetone, Amber Glass Bottles,
More...
|
| Quality Level | 200 |
| grade | ACS reagent |
| vapor density | 2 (vs air) |
| vapor pressure | 184 mmHg ( 20 °C) |
| assay | ≥99.5% |
| form | liquid (clear) |
| shelf life | Recommended retest period - 2 years |
| expl. lim. | 13.2 % |
| impurities | ≤0.0003 meq/g Titr. acid |
| ≤0.0006 meq/g Titr. base | |
| ≤0.002% aldehyde as formaldehyde | |
| ≤0.05% isopropanol | |
| ≤0.05% methanol | |
| ≤0.5% water | |
| evapn. residue | ≤0.001% |
| color | APHA: ≤10 |
| refractive index | n20/D 1.359 (lit.) |
| bp | 56 °C/760 mmHg (lit.) |
| mp | −94 °C (lit.) |
| density | 0.791 g/mL at 25 °C (lit.) |
| SMILES string | CC(C)=O |
| InChI | 1S/C3H6O/c1-3(2)4/h1-2H3 |
| InChI key | CSCPPACGZOOCGX-UHFFFAOYSA-N |
General description
Acetone is a polar organic solvent. It can undergo photocatalytic oxidation in the presence of mixed TiO2-rare earth oxides.
Safety Information


Application
Acetone may be used in the synthesis of Ga (Gallium)-DOTATATE (where DOTA= 1,4,7,10-tetraazacyclo- dodecane -1,4,7,10-tetraacetic acid) chemicals.6 It may be used in an assay for the determination of ester groups in lipids by spectrophotometric methods.[4]
Acetone undergoes aldolization in the presence of Mg-Al layered double hydroxides (LDH) as catalysts and Cl- and/or CO32- as compensating anions to afford diacetone alcohol and mesityl oxide as the main products.[2] Its enantioselective Aldol condensation with various isatins in the presence of a dipeptide catalyst forms 1-alkyl 3-(2-oxopropyl)-3-hydroxyindolin-2-ones.[3] Aqueous solution of acetone may be used as a medium for the oxidation of alkynes to 1,2-diketones using potassium permanganate.[5]
Acetone′s luminesence intensity is dependent upon the solution components . The absorption of UV light by acetone, results in its photolysis and the production of radials .
Packaging
1, 6×1, 2.5, 4×2.5, 4, 4×4 L in glass bottle
18, 20 L in steel drum
200 L in Pure-Pac™ 1
4×4 L in poly bottle
View returnable container options.
200 L in steel drum
500, 6×500 mL in glass bottle
Other Notes
Go to our BioRenewable Alternative Acetone - 904082
For information on acetone miscibility, please visit the following link:
Acetone Miscibility/Immiscibility Table![]()
Pure-Pac® II containers require the Micromatic MacroValve coupler for dispensing solvents, Z560723.
Legal Information
Pure-Pac is a registered trademark of Sigma-Aldrich Co. LLC