Description
Trifluoroacetic acid
ReagentPlus®, 99%
Synonym(s):
TFA
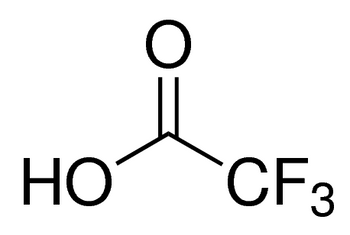
Linear Formula:
CF3COOH
CAS Number:
76-05-1
Molecular Weight:
114.02
Beilstein:
742035
EC Number:
200-929-3
MDL number:
MFCD00004169
PubChem Substance ID:
329826846
NACRES:
NA.21
General description
Trifluoroacetic acid (TFA) is an organofluorine compound used as a reagent in organic synthesis for various acid-catalyzed reactions such as ring-opening of epoxides, biomimetic cyclization, Cope rearrangements, and natural product synthesis. TFA′s physicochemical characteristics provide advantages over other acids because of its high volatility, solubility in organic solvents, and acidic strength. When TFA is used as a reagent the product isolation is simple by evaporation due to its very high volatility. Less volatile acids such as sulfuric acid or p-toluenesulfonic acid may require neutralization or an extractive workup.[1]
Application
Trifluoroacetic acid can be used as a reagent:
TFA can also be used as:
- For the cleavage of nitrogen and oxygen protecting groups such as N-Boc, N-benzyloxymethyl, benzyl ether, p-methoxybenzyl ether, t-butyl ether, t-butyloxymethyl ether, triphenylmethyl ether, and dimethyl acetals.[1][2][3]
- In the Baeyer–Villiger oxidation reactions in combination with sodium percarbonate.[1],·
- For the C-H trifluoromethylation of arenes.[4]
TFA can also be used as:
- A solvent in atom transfer cyclization reactions and polymer processes.[2]
- A catalyst in the synthesis of ε-caprolactam via Beckmann rearrangement of cyclohexanone oxime in aprotic solvents.[5]
Packaging
1mL in each ampule.
Legal Information
ReagentPlus is a registered trademark of Sigma-Aldrich Co. LLC
SAFETY INFORMATION
Pictograms
GHS05,GHS07
Signal Word
Danger
Hazard Statements
H314 - H332 - H412
Precautionary Statements
P261 - P273 - P280 - P303 + P361 + P353 - P304 + P340 + P310 - P305 + P351 + P338
Hazard Classifications
Acute Tox. 4 Inhalation - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1A
Storage Class Code
8A - Combustible, corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
212.0 °F - Pensky-Martens closed cup
Flash Point(C)
> 100 °C - Pensky-Martens closed cup