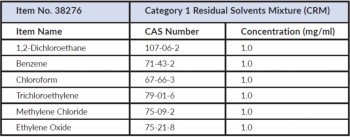
Description
Residual Solvents Mixture - Class IIC
Pharmaceutical Secondary Standard; Certified Reference Material
| Related Categories | Additional Standards, Analytical Standards, Analytical/Chromatography, Application Areas and Market Segments, Chromatography,
I-Z, Method and Regulation Specific, Neats and Solutions, Pharma, Pharmaceutical Secondary Standards, Pharmaceutical and Drug Standards, Pharmacopeia & Metrological Institutes Standards, Residual Solvent Standards, Residual Solvents Standards, Residue Solvents, Secondary Pharmaceutical Standards, USP
|
| grade | certified reference material |
| pharmaceutical secondary standard | |
| suitability | suitable for 467 per USP |
| Featured Industry | Environmental Pharmaceutical (small molecule) |
| pharmacopeia traceability | traceable to USP 1601306 |
General description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Analysis Note
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
Other Notes
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
|
Description |
|---|
| N,N-Dimethylacetamide 5.45 mg/mL |
| N,N-Dimethylformamide 4.4 mg/mL |
| 2-Ethoxyethanol .8 mg/mL |
| Ethylene glycol 3.1 mg/mL |
| Formamide 1.1 mg/mL |
| 2-Methoxyethanol .25 mg/mL |
| N-Methylpyrrolidone 2.65 mg/mL |
| Sulfolane .8 mg/mL |
Safety Information
Signal word
Danger
Hazard statements
H360
Precautionary statements
P201-P280-P308 + P313
RIDADR
NONH for all modes of transport
WGK Germany
1
Flash Point(F)
188.6 °F
Flash Point(C)
87 °C