Description
DL-Malic acid
meets analytical specification of FCC, E296, 99-100.5% (alkalimetric)
Synonym: (±)-2-Hydroxysuccinic acid, DL-Hydroxybutanedioic acid
-
CAS Number 6915-15-7
-
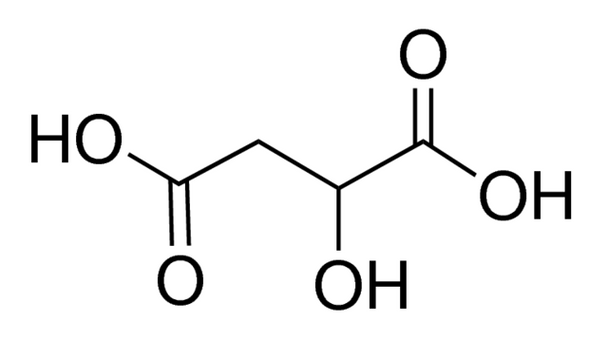
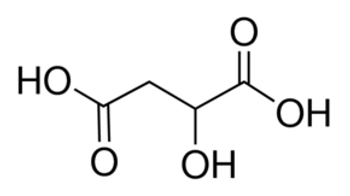
Linear Formula HO2CCH2CH(OH)CO2H
-
Molecular Weight 134.09
-
Beilstein/REAXYS Number 1723539
-
EC Number 230-022-8
-
MDL number MFCD00064212
-
PubChem Substance ID 57648231

-
NACRES NA.25
Properties
| Related Categories | Analytical/Chromatography, Pharmacopoeia, Pharmacopoeia A-Z Less... |
| vapor density | 4.6 (vs air) |
| vapor pressure | <0.1 mmHg ( 20 °C) |
| assay | 99-100.5% (alkalimetric) |
| optical activity | [α]/D −0.10 to +0.10° |
| autoignition temp. | 644 °F |
| quality | meets analytical specification of FCC, E296 |
| impurities | ≤0.05% maleic acid |
| x | ≤0.1% water insoluble matter |
| x | ≤1% fumaric acid |
| ign. residue | ≤0.1% (as SO4) |
| mp | 129-133 °C |
| x | 131-133 °C (lit.) |
| solubility | acetone: soluble 17.75 g/ 100 gm at 20 °C |
| x | diethyl ether: soluble 0.84 g/100 gm at 20 °C |
| x | dioxane: soluble 22.70 g/100 gm at 20 °C |
| x | ethanol: soluble 45.53 g/100 gm at 20 °C |
| x | methanol: soluble 82.70 g/100 g at 20 °C |
| x | water: soluble 55.8 g/100 gm at 20 °C |
| x | benzene: insoluble |
| cation traces | As: ≤3 mg/kg |
| x | Hg: ≤1 mg/kg |
| x | Pb: ≤2 mg/kg |
| suitability | complies for identity (IR) |
| Featured Industry | Pharmaceutical (small molecule) |
| SMILES string | OC(CC(O)=O)C(O)=O |
| InChI | 1S/C4H6O5/c5-2(4(8)9)1-3(6)7/h2,5H,1H2,(H,6,7)(H,8,9) |
| InChI key | BJEPYKJPYRNKOW-UHFFFAOYSA-N |
Application
DL-Malic acid is an organic acid used for studying the impact on ultrasound pasteurization.[2] It has also been used to culture Pichia membraneafaciens.[3]
Biochem/physiol Actions
Malic acid is a dicarboxylic acid and an important regulatory metabolite. It has been implicated in process of fruit ripening. Malic acid is important for the starch metabolism; low malic acid content results in transient accumulation of starch. Mitochondrial-malate metabolism modulates ADP-glucose pyrophosphorylase activity and redox status of plastids.[1]
Safety Information