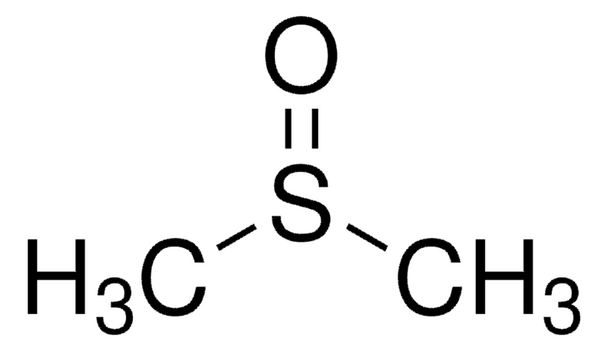
Description
Dimethyl sulfoxide
puriss. p.a., ACS reagent, ≥99.9% (GC)
Synonym(s):
DMSO
Linear Formula:
(CH3)2SO
CAS Number:
67-68-5
Molecular Weight:
78.13
Beilstein:
506008
EC Number:
200-664-3
MDL number:
MFCD00002089
PubChem Substance ID:
329756130
NACRES:
NA.21
PROPERTIES
grade
ACS reagent
puriss. p.a.
vapor density
2.7 (vs air)
vapor pressure
0.42 mmHg ( 20 °C)
Assay
≥99.9% (GC)
form
liquid
autoignition temp.
573 °F
expl. lim.
42 %, 63 °F
impurities
≤0.001% free acid (as CH3COOH)
≤0.1% water
evapn. residue
≤0.005%
refractive index
n20/D 1.479 (lit.)
n20/D 1.479
bp
189 °C (lit.)
mp
16-19 °C (lit.)
18-20 °C
density
1.10 g/mL (lit.)
cation traces
Al: ≤0.5 mg/kg
Ba: ≤0.1 mg/kg
Bi: ≤0.1 mg/kg
Ca: ≤0.5 mg/kg
Cd: ≤0.05 mg/kg
Co: ≤0.02 mg/kg
Cr: ≤0.02 mg/kg
Cu: ≤0.02 mg/kg
Fe: ≤0.5 mg/kg
K: ≤0.5 mg/kg
Li: ≤0.1 mg/kg
Mg: ≤0.1 mg/kg
Mn: ≤0.02 mg/kg
Mo: ≤0.1 mg/kg
Na: ≤1 mg/kg
Ni: ≤0.5 mg/kg
Pb: ≤0.1 mg/kg
Sr: ≤0.1 mg/kg
Zn: ≤0.1 mg/kg
SMILES string
CS(C)=O
InChI
1S/C2H6OS/c1-4(2)3/h1-2H3
InChI key
IAZDPXIOMUYVGZ-UHFFFAOYSA-N
General description
Dimethyl sulfoxide (DMSO) is a polar solvent.[1] DMSO activated by electrophile (oxalyl chloride) has been used to oxidize various alcohols (primary, secondary, allylic, benzylic, hindered and bicyclic). Carbonyl compounds are formed as reaction products.[2] It has been proposed as an alternate solvent for methyl cellosolve for use in ninhydrin reaction, due to its high stability.[3]
Caution
Supercools easily and remelts slowly at room temperature. Solidified product can be re-liquified by warming to room temperature without detriment to the product.
SAFETY INFORMATION
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
188.6 °F - closed cup
Flash Point(C)
87 °C - closed cup