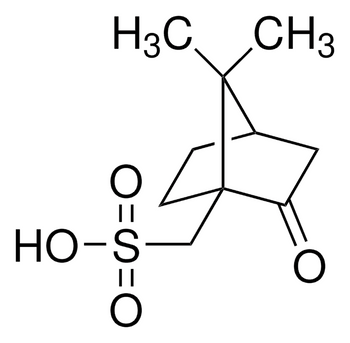
Description
(1R)-(−)-10-Camphorsulfonic acid, 98%
Synonym(s):
(−)-Camphor-10-sulfonic acid, (1R)-(−)-Camphor-10-sulfonic acid, (1R)-Camphor-10-sulfonic acid
Empirical Formula (Hill Notation):
C10H16O4S
CAS Number:
35963-20-3
Molecular Weight:
232.30
Beilstein:
2809676
EC Number:
252-817-9
MDL number:
MFCD00064158
PubChem Substance ID:
24857020
NACRES:
NA.22
PROPERTIES
Quality Level
100
Assay
98%
optical activity
[α]20/D −21°, c = 2 in H2O
mp
198 °C (dec.) (lit.)
SMILES string
[H][C@]12CC[C@](CS(O)(=O)=O)(C(=O)C1)C2(C)C
InChI
1S/C10H16O4S/c1-9(2)7-3-4-10(9,8(11)5-7)6-15(12,13)14/h7H,3-6H2,1-2H3,(H,12,13,14)/t7-,10-/m0/s1
InChI key
MIOPJNTWMNEORI-XVKPBYJWSA-N
General description
(1R)-(−)-10-Camphorsulfonic acid is an HPLC derivatization reagent for UV/Vis detection. It is mainly employed for the resolution of bases.
Application
(1R)-(−)-10-Camphorsulfonic acid may be used as a chiral building block for the synthesis of pentavalent organo-bismuth derivatives by enantioselective C-arylation.[1] It may be used as a chiral monomer in the enantioselective sensing of chiral amino acids by potentiometric sensors based on optical active polyaniline films.[2]
SAFETY INFORMATION
Pictograms
GHS05
Signal Word
Danger
Hazard Statements
H314
Precautionary Statements
P260 - P280 - P303 + P361 + P353 - P304 + P340 + P310 - P305 + P351 + P338 - P363
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible, corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves