Description
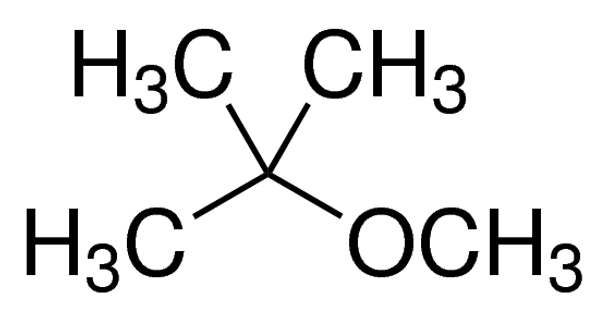
tert-Butyl methyl ether
ACS reagent, ≥99.0%
Synonym: MTBE, Methyl tert-butyl ether
-
CAS Number 1634-04-4
-
Linear Formula (CH3)3COCH3
-
Molecular Weight 88.15
-
Beilstein/REAXYS Number 1730942
-
EC Number 216-653-1
-
MDL number MFCD00008812
-
PubChem Substance ID 329756503
-
NACRES NA.07
Properties
| Related Categories | ACS Grade, ACS Grade Solvents, ACS and Reagent Grade Solvents, Amber Glass Bottles, Carbon Steel Cans with NPT Threads,
Closed Head Drums, Drums Product Line, Semi-Bulk Solvents, Solvent Bottles, Solvent Packaging Options, Solvent by Application, Solvents, Sure/Seal Bottles
Less... |
| Quality Level | 200 |
| grade | ACS reagent |
| vapor pressure | 4.05 psi |
| vapor density | 3.1 (vs air) |
| assay | ≥99.0% |
| autoignition temp. | 705 °F |
| expl. lim. | 15.1 % |
| impurities | ≤0.05% water |
| - | <1 ppm H2O2 |
| evapn. residue | ≤0.001% |
| color | APHA: ≤10 |
| refractive index | n20/D 1.369 (lit.) |
| bp | 55-56 °C (lit.) |
| mp | -110 °C |
| density | 0.74 g/mL at 25 °C (lit.) |
| SMILES string | COC(C)(C)C |
| InChI | 1S/C5H12O/c1-5(2,3)6-4/h1-4H3 |
| InChI key | BZLVMXJERCGZMT-UHFFFAOYSA-N |
General description
tert-Butyl methyl ether (methyl tert-butyl ether, MTBE) is a gasoline additive. Its oxidative degradation by propane-oxidizing bacterial strains has been tested.[1] Kinetics of its heat-mediated biodegradation has been investigated under various reaction conditions. Its biodegradation followed a pseudo-first-order decay model and its pseudo-first-order rate constants were evaluated.[2] Its extraction from water has been proposed by employing an organic polymeric compound, Nafion.[3] MTBE can be synthesized by acid-catalyzed reaction between methanol and isobutene.[5] A study suggests that the addition of MTBE increases the number of active sites during polymerization of propene by stopped-flow method.[6] This solvent meets ACS (American Chemical Society) specifications and can be used for processes requiring strict quality conditions such as analytical testing.
Application
tert-Butyl methyl ether (methyl tert-butyl ether, MTBE) may be used:
• As sole carbon and energy source in a mixed microbial consortium.[4]
• To extract lipids from biological fluids for shotgun profiling.[7]
• To synthesize fatty acid methyl esters (FAMEs) and glycerol tert-butyl ether by transesterification of canola oil in the absence of catalyst under supercritical condition.[8]
Packaging
1, 6×1 L in Sure/Seal
2.5, 4×2.5, 4×4 L in glass bottle
18, 200 L in steel drum
500 mL in Sure/Seal