Description
Salmonella arizonae, choleraesuis subspecies arizonae, ATCC 13314, DuoPak (KWIK-STIK 2 Pack, with built in hydrating fluid), ATCC Licensed Derivative, by Microbiologics
DUO-PAK™ Two ready-to-use swabs that contain a single lyophilized pellet of a single microorganism strain. Rehydrating fluid is built into the Kwik-Stik unit. These microorganisms are reference stock cultures that have been derived from reference cultucollections such as American Type Culture Collection.
To use, simply crush the rehydrating ampule, mix, remove swab, and inoculate the agar plate.
Each shipment includes a Certificate of Analysis, MSDS, and complete instructions. All shipments are drop-shipped directly from the manufacturer. A hazmat charge will be applied.
SUMMARY AND PRINCIPLE
A reliable source of reference stock cultures for use in Microbiology Quality Assurance programs is essential. Microorganisms with known and predictable growth requirements, selective and differential properties, biochemical activity or phenotypic profiles, serological, assay responses, antimicrobial susceptibility characteristics, and assay values are used in Quality Control, education and proficiency programs.
Ultra-cold freezing and lyophilization are well-documented methods for preserving microorganisms. LYFO DISK ® and KWIK-STIK™ Microorganisms incorporate a lyophilization method, reported by Obara et. al., which uses a suspending medium consisting of gelatin, skim milk, ascorbic acid, dextrose and charcoal. The gelatin serves as a carrier for the microorganism. Skim milk, ascorbic acid, and dextrose protect the microorganism by preserving the integrity of the cell wall during freeze-drying and storage. The charcoal is included to neutralize any toxic substances formed during the lyophilization process.
LYFO DISK ® and KWIK-STIK™ Microorganisms are derivatives and are traceable to authentic reference culture collections such as the American Type Culture Collection (ATCC ® ).
SPECIFICATIONS AND PERFORMANCE
The production and process design for these lyophilized microorganism preparations result in a pure population of an individual microorganism that is traceable to an authentic reference culture.
Quality Assurance documentation includes, but is not limited to, the product label and a Certificate of Analysis stating:
- the authenticity and traceability of the microorganism preparation to a reference culture, and;
- the number of passages the microorganism preparation has been removed from the reference culture.
The Certificate of Analysis (attached to the Packing Slip) is included in each microorganism shipment.
PRODUCT DESCRIPTION
A. LYFO DISK ® Microorganisms
LYFO DISK ® Microorganisms are packaged in a resealable vial that contains ten (10) lyophilized pellets of a single microorganism strain and a desiccator to prevent adverse moisture accumulation.
B. KWIK-STIK™ Microorganisms
Each KWIK-STIK™ unit contains a lyophilized pellet of a single microorganism strain, a reservoir of hydrating fluid and an inoculating swab. Each device is sealed within a laminated pouch that contains a desiccator to prevent adverse moisture accumulation. KWIK-STIK™ Microorganisms are available as
- ten (10) KWIK-STIK™ devices of a single microorganism strain in the Canister configuration, or
- two (2) KWIK-STIK™ devices of a single microorganism strain in the DuoPak configuration.
C. MICROORGANISM SETS
Microorganism Sets are combinations of LYFO DISK ® or KWIK-STIK™ Microorganisms that are used together for common applications.
STORAGE AND EXPIRATION
Store the LYFO DISK ® and KWIK-STIK™ Microorganisms at 2-8 degrees C. in the dark, in the original, sealed pouch or vial containing the desiccator. The lyophilized microorganism may be rendered nonviable by an abnormal accumulation of moisture in the pellet, or excessive heat, due to improper storage or handling.
The LYFO DISK ® and KWIK-STIK™ Microorganisms should not be used if:
- the original seal has been broken;
- there is evidence of excessive exposure to heat or moisture;
- the expiration date has passed.
PRECAUTIONS AND LIMITATIONS
These products are for laboratory use and is to be used only by adequately trained and qualified laboratory personnel. Observe approved biohazard precautions and aseptic techniques. All laboratory specimens should be considered infectious and handled according to "standard precautions".
For additional information regarding specific precautions for the prevention of the transmission of all infectious agents from laboratory instruments and materials, and for recommendations for the management of exposure to infectious disease, refer to CLSI document M-29: Protection of Laboratory Workers from Occupationally Acquired Infections: Approved Guideline.
Sterilize all biohazard waste before disposal.
Refer to the keyword "Precautions", in the Hardy Diagnostics software program HUGO™, for more information regarding general precautions when using culture media.
Refer to the keyword "MSDS", in the Hardy Diagnostics software program HUGO™, for more information on handling potentially hazardous material.
IMPORTANT CONSIDERATIONS
Lyophilized microorganisms are no different than naturally occurring strains with regard to their requirements for nutrients, specific supplements, incubation temperatures, or environment demands.
- A non-selective, nutrient or enriched agar medium should be selected for the primary growth.
- Optimal incubation temperature and environmental conditions should be selected for the primary growth.
- Fluid medium must never be used for primary growth. Because of the manipulations required during hydration, it is difficult to obtain purity of a lyophilized strain in a fluid medium. A contaminant may completely overgrow and obscure the presence of the lyophilized strain.
- The 0.5ml volume of hydrated material accommodates the inoculation of multiple, non-selective agar media. The inoculated plates can then be distributed to point-of-care laboratories.
- Because of the similarity of the KWIK-STIK™ device to various collection and transportation devices, never remove the "ETIOLOGICAL AGENTS" label from the device.
INSTRUCTIONS FOR USE
LYFO DISK ® Microorganisms
1. Remove the LYFO DISK ® vial from 2-8 degrees C. storage and allow the unopened vial to equilibrate to room temperature.
2. Using sterile forceps, aseptically remove one (1) gelatin pellet from the vial. Do not remove the desiccant. Place the pellet in 0.5ml of sterile Tryptic Soy Broth, Brain Heart Infusion Broth, saline, or deionized water. Immediately stopper and recap vial and return to 2-8 degrees C. storage.
3. Emulsify and crush pellet with a sterile swab until the pellet particles are uniform in size and the suspension is homogenous in appearance. DO NOT INCUBATE.
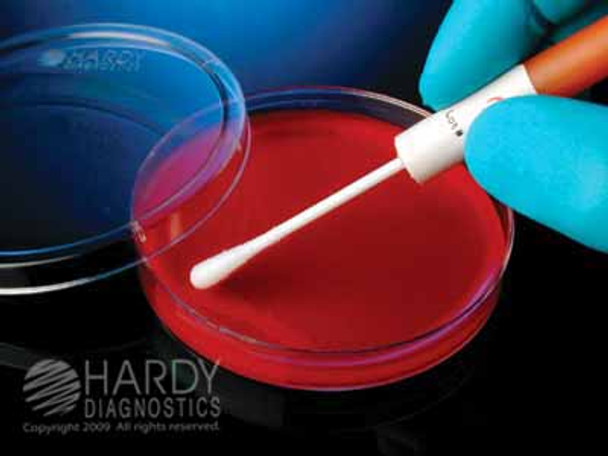
4. IMMEDIATELY , saturate the swab with the hydrated material and transfer the material to an appropriate, non-selective, nutrient or enriched agar medium. With pressure, rotate the swab, and inoculate a circular area (i.e. one inch or 25mm in diameter) of the agar medium. Using the same swab or a sterile loop, repeatedly (about 10 to 20 times) streak through the inoculated area and then continue to streak the remainder of the agar surface.
5. Release the hydrating fluid by breaking the ampoule using a pinching action at the middle of the ampoule in the cap of the device. Allow the hydrating fluid to flow through the swab shaft and INTO the bottom portion of the unit containing the gelatin pellet.
6. IMMEDIATELY , incubate the inoculated media at temperature and atmospheric conditions that are appropriate for the microorganism.
7. Following incubation, select representative well-isolated colonies for indicated transfers.
KWIK-STIK™ Microorganisms
1. Remove the KWIK-STIK™ unit from 2-8 degrees C. storage and allow the unopened pouch to equilibrate to room temperature.
2. Open the pouch and remove the KWIK-STIK™ unit.
3. Tear off the pull tab portion of the label from the KWIK-STIK™ device. The label can be attached to permanent QC Records or attached to the primary plate agar medium for identification.
4. Take note of the position of the gelatin pellet in the bottom part of the device and the reservoir of hydrating fluid in the top (cap) part of the device. DO NOT DISASSEMBLE THE DEVICE DURING HYDRATION.
5. Release the hydrating fluid by pinching the very top of the ampule in the cap of the device. Allow the hydrating fluid to flow through the swab shaft and into the bottom portion of the unit containing the gelatin pellet.
6. Holding the device vertically, with the cap up, and tapping the bottom of the device on the counter can further facilitate the flow of the fluid.
7. Using a pinching action on the bottom portion of the unit, crush and mix the pellet in the fluid until the pellet particles are uniform in size and the suspension is homogenous in appearance. DO NOT INCUBATE.
8. Now that the swab is saturated with the hydrated material, immediately transfer the material to an appropriate, non-selective, nutrient or enriched agar medium. With pressure, rotate the swab and inoculate a circular area (i.e. one inch or 25mm in diameter) of the agar medium. With a sterile loop, streak repeatedly through the inoculated area in order to obtain isolated colonies.
9. Discard the remaining hydrated material in accordance with the laboratory protocol for disposal of biohazardous materials.
10. IMMEDIATELY, incubate the inoculated media at temperature and atmospheric conditions that are appropriate for the microorganism.
11. Following incubation, select representative well-isolated colonies for indicated transfers.
QUALITY CONTROL
This product is produced in compliance with the FDA: Quality System Regulation; 1996 (QSR). Quality Control functions include, but are not limited to, purity, growth characteristics, morphological features and biochemical activity. The decision to perform additional in-house Quality Control is the responsibility of each individual laboratory.
MATERIALS REQUIRED BUT NOT PROVIDED
- LYFO DISK ® Microorganisms require 0.5ml of sterile Tryptic Soy Broth (Cat. no. R36), Brain Heart Infusion Broth (Cat. no. R10), saline, or deionized water to hydrate the lyophilized preparation.
- LYFO DISK ® and KWIK-STIK™ Microorganisms both require non-selective, nutrient or enriched agar media to optimize growth and recovery.
- LYFO DISK ® and KWIK-STIK™ Microorganisms both require specific incubation time and conditions to optimize growth and recovery.