Description
DTT
1,4-Dithiothreitol
Synonym: 1,4-dithiothreitol, threo-1,4-Dimercapto-2,3-butanediol, DL-Dithiothreitol, Cleland’s reagent, DTT, cleland′s reagent, dithiothreitol, 1,4-
-
CAS Number 3483-12-3
-
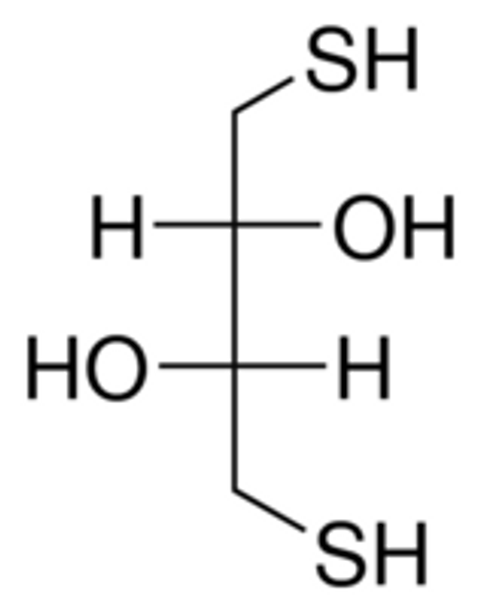
Linear Formula HSCH2CH(OH)CH(OH)CH2SH
-
Molecular Weight 154.25
-
Beilstein Registry Number 1719757
-
MDL number MFCD00004877
Properties
| assay | ≥97% (Ellman′s reagent) |
| form | crystalline powder |
| mol wt | Mr 154.3 |
| packaging | pkg of 10 g (10708984001) |
| - | pkg of 2 g (10197777001) |
| - | pkg of 25 g (11583786001) |
| mfr. no. | Roche |
| mp | 41-44 °C(lit.) |
| shipped in | wet ice |
| storage temp. | 2-8°C |
Description
Application
An excellent reagent for maintaining SH groups in reduced state; quantitatively reduces disulfides. DTT is effective in sample buffers for reducing protein disulfide bonds prior to SDS-PAGE. DTT can also be used for reducing the disulfide bridge of the cross-linker N,N′-bis(acryloyl)cystamine to break apart the matrix of a polyacrylamide gel. DTT is less pungent and is less toxic than 2-mercaptoethanol. Typically, a seven fold lower concentration of DTT (100 mM) is needed than is used for 2-mercaptoethanol (5% v/v, 700 mM).
1,4-dithiothreitol is used in:
• Isolation, purification and characterization of proteins and enzymes
• Measurement of enzyme activities (reactivation of enzymes)
• Determination of disulfide groups in proteins and enzymes
• DNA extraction prior to amplification
Other Notes
For life science research only. Not for use in diagnostic procedures.
Preparation Note
Storage conditions (working solution): A solution of DTT in Hepes buffer, pH 7.75 is stable for one week at 2 to 8 °C if the container is tightly sealed and the solution is protected from atmospheric oxygen by argon or nitrogen.
General description
1,4-dithiothreitol is commonly known as Cleland′s reagent. It confers protection to thiol groups and reduces disulfide bonds in peptides and proteins. It reduces disulfide bonds to sulfhydryl group and is commonly used in receptor studies, to determine the functional importance of disulfide bonds during receptor occupancy and functionality.
Specifications
Oxidized form: <2.5% (absorbance at 283nm)
Sequence
Enantiomers
Our DTT-preparation is optically inactive, i.e. it is the D,L-DTT.