Description
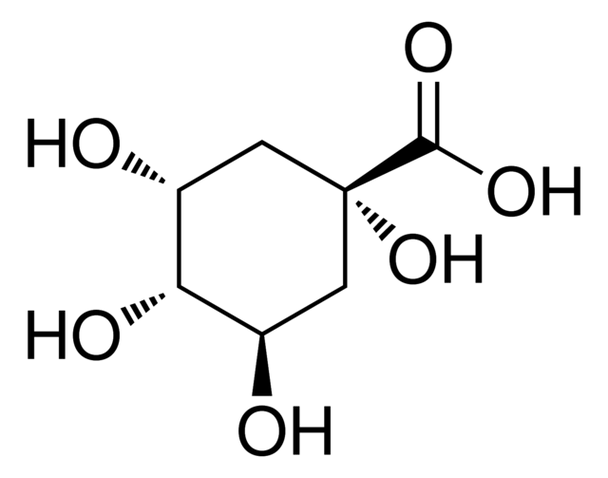
D-(−)-Quinic acid, 98%, 100G
Empirical Formula (Hill Notation):
C7H12O6
CAS Number:
77-95-2
Molecular Weight:
192.17
Beilstein:
2212412
EC Number:
201-072-8
MDL number:
MFCD00003864
PubChem Substance ID:
24848361
NACRES:
NA.22
Quality Level
200
assay
98%
form
powder
optical activity
[α]20/D −43.9°, c = 11.2 in H2O
SMILES string
O[C@@H]1C[C@@](O)(C[C@@H](O)[C@H]1O)C(O)=O
InChI
1S/C7H12O6/c8-3-1-7(13,6(11)12)2-4(9)5(3)10/h3-5,8-10,13H,1-2H2,(H,11,12)/t3-,4-,5-,7+/m1/s1
InChI key
AAWZDTNXLSGCEK-WYWMIBKRSA-N
General description
D-(-)-Quinic acid, a plant metabolite, is chiral building block used in multistep chemical synthesis of natural compounds.
Application
D-(−)-Quinic acid can be used as:
- A chiral selector electrolyte along with copper(II) sulfate. This electrolyte is utilized in chiral resolution DL-tartaric acid by ligand-exchange capillary electrophoresis method.
- A starting material in the synthesis of stereoisomers of 3,4,6-trihydroxyazepanes, 7-hydroxymethyl-3,4,5-trihydroxyazepanes, and 3,4,5-trihydroxyazepanes, as potential inhibitors of glycosidase.
- A precursor for the preparation of trihydroxy piperidine derivatives and (+)-proto-quercitol glycosidase inhibitors.
D-(-)-Quinic acid has been used as a standard to determine the composition of organic acids in bitter gentian teas and in developing cranberry fruit by HPLC. It may be used in the preparation of 3,4-O-isopropylidene-3(R),4(S)-dihydroxycyclohexanone.
Packaging
25, 100 g in poly bottle
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves