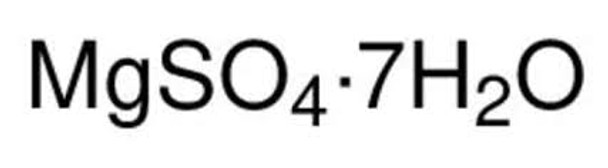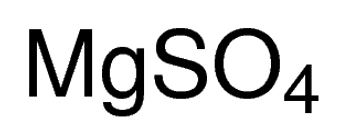Description
Magnesium sulfate heptahydrate, 2.5kg
ACS reagent, ≥98%
Synonym(s):
Epsom salts
Linear Formula:
MgSO4 · 7H2O
CAS Number:
10034-99-8
Molecular Weight:
246.47
EC Number:
231-298-2
MDL number:
MFCD00149785
eCl@ss:
38030409
PubChem Substance ID:
329752370
NACRES:
NA.21
PROPERTIES
grade
ACS reagent
Quality Level
200
vapor density
<0.01 (vs air)
vapor pressure
<0.1 mmHg ( 20 °C)
Assay
≥98%
98.0-102.0% (ACS specification)
form
crystals
impurities
≤0.005% insolubles
pH
5.0-8.2 (25 °C, 5%)
anion traces
chloride (Cl-): ≤5 ppm
nitrate (NO3-): ≤0.002%
cation traces
Ca: ≤0.02%
Fe: ≤5 ppm
K: ≤0.005%
Mn: ≤5 ppm
NH4+: ≤0.002%
Na: ≤0.005%
Sr: ≤0.005%
heavy metals: ≤5 ppm (by ICP-OES)
SMILES string
O.O.O.O.O.O.O.[Mg++].[O-]S([O-])(=O)=O
InChI
1S/Mg.H2O4S.7H2O/c;1-5(2,3)4;;;;;;;/h;(H2,1,2,3,4);7*1H2/q+2;;;;;;;;/p-2
InChI key
WRUGWIBCXHJTDG-UHFFFAOYSA-L
DESCRIPTION
General description
Magnesium sulfate heptahydrate is a hydrated salt of magnesium. It is commonly used as a catalyst in organic synthesis.
Application
Magnesium sulfate heptahydrate can be used as a Lewis acid catalyst to synthesize:
NaBH4 supported on MgSO4 7H2O can be used as an efficient catalyst for the reduction of α, β-unsaturated carbonyl compounds to corresponding alcohols.
- Phenazine and quinoxaline derivatives via a condensation reaction between o-phenylenediamines and 1,2-dicarbonyl compounds.
- Imidazole derivatives via one-pot multicomponent condensation reaction of aromatic aldehydes, benzil, aliphatic, and aromatic primary amines.
NaBH4 supported on MgSO4 7H2O can be used as an efficient catalyst for the reduction of α, β-unsaturated carbonyl compounds to corresponding alcohols.
SAFETY INFORMATION
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable