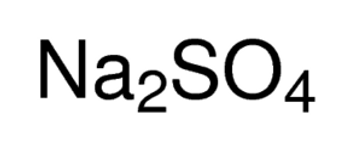Magnesium sulfate anhydrous, reagent grade, (2.5 kg)
Description
Magnesium sulfate anhydrous, reagent grade, 97% (2.5 kg)
Empirical Formula (Hill Notation):
MgSO4
CAS Number:
7487-88-9
Molecular Weight:
120.37
EC Number:
231-298-2
MDL number:
MFCD00011110
PubChem Substance ID:
329752038
NACRES:
NA.21
PROPERTIES
grade
anhydrous
reagent grade
Quality Level
100
vapor density
<0.01 (vs air)
vapor pressure
<0.1 mmHg ( 20 °C)
Assay
≥97%
form
granular
pH
~7.9 (25 °C, 50 g/L)
SMILES string
[Mg++].[O-]S([O-])(=O)=O
InChI
1S/Mg.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)/q+2;/p-2
InChI key
CSNNHWWHGAXBCP-UHFFFAOYSA-L
DESCRIPTION
Application
Magnesium sulfate (MgSO4) has been used as a medium supplement in the M63 minimal medium for bacteria.[1]
Anhydrous MgSO4 plays the role of a drying agent in the synthesis of 3,6-bis(3,4′-dihexyl-2,2′-bithiophen-5-yl)-1,2,4,5-tetrazine[2] and organic molecules having an A–D–A (D = donor, A = acceptor) structure.[3]
It may be used as an additional reagent in the synthesis of t-butyl esters and ethers by reacting carboxylic acid or alcohol and t-butanol in the presence of catalytic sulfuric acid.[4]
Anhydrous MgSO4 plays the role of a drying agent in the synthesis of 3,6-bis(3,4′-dihexyl-2,2′-bithiophen-5-yl)-1,2,4,5-tetrazine[2] and organic molecules having an A–D–A (D = donor, A = acceptor) structure.[3]
It may be used as an additional reagent in the synthesis of t-butyl esters and ethers by reacting carboxylic acid or alcohol and t-butanol in the presence of catalytic sulfuric acid.[4]
SAFETY INFORMATION
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable