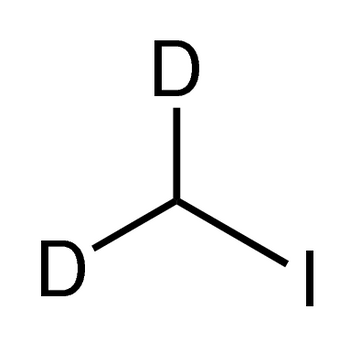
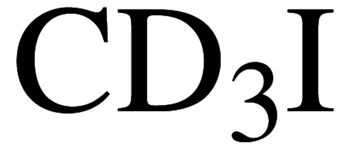Description
Iodomethane
purum, ≥99.0% (GC)
Synonym(s):
Methyl iodide
About This Item
Empirical Formula (Hill Notation):
CH3I
CAS Number:
74-88-4
Molecular Weight:
141.94
Beilstein/REAXYS Number:
969135
EC Number:
200-819-5
MDL number:
MFCD00001073
UNSPSC Code:
12352100
PubChem Substance ID:
329760660
NACRES:
NA.21
Properties
vapor density
4.89 (vs air)
Quality Level
200
vapor pressure
24.09 psi ( 55 °C)
7.89 psi ( 20 °C)
grade
purum
assay
≥99.0% (GC)
contains
silver wool as stabilizer
impurities
≤0.5% CH2I2
refractive index
n20/D 1.530
n20/D 1.531 (lit.)
bp
41-43 °C (lit.)
41-43 °C
mp
−64 (lit.)
solubility
water: soluble 14 g/L at 20 °C
density
2.28 g/mL at 25 °C (lit.)
SMILES string
CI
InChI
1S/CH3I/c1-2/h1H3
InChI key
INQOMBQAUSQDDS-UHFFFAOYSA-N
General description
Iodomethane (Methyl iodide) is a colorless liquid. It can be prepared by reacting dimethyl sulfate with concentrated KI. Reaction of iodine and phosphorous with methanol has been reported to afford iodomethane.[1] It reacts with tris(triphenylphosphine)chlororhodium(I) to afford the octahedral complex of rhodium(III), [RhlCl(CH3)(PPh3)2(ICH3)].[2] Kinetics of its reaction with atomic chlorine in the gas phase has been investigated. The absolute rate constant of the reaction has been reported.[3]
Application
Iodomethane (Methyl iodide) may be employed in the preparation of stable neutral solutions of hypoiodous acid[4] and various methyl esters.[5]
Safety Information
pictograms
GHS02,GHS06,GHS08,GHS05,GHS09
signalword
Danger
hcodes
H226,H301 + H331,H312,H315,H318,H335,H351,H410
pcodes
P210 - P273 - P280 - P301 + P310 - P303 + P361 + P353 - P305 + P351 + P338
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Acute 1 - Aquatic Chronic 2 - Carc. 2 - Eye Dam. 1 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
wgk_germany
WGK 3
ppe
Faceshields, Gloves, Goggles