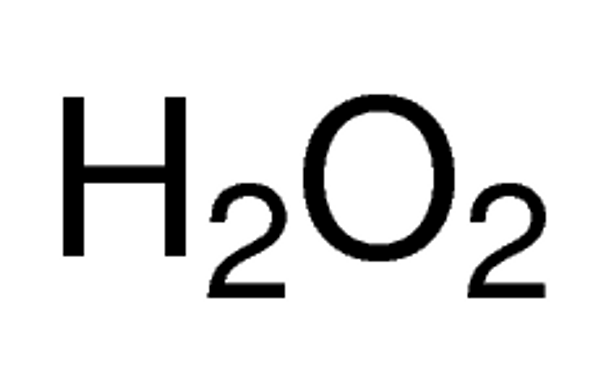Description
Hydrogen peroxide solution
50 wt. % in H2O, stabilized
-
CAS Number 7722-84-1
-
Empirical Formula (Hill Notation) H2O2
-
Molecular Weight 34.01
-
Beilstein/REAXYS Number 3587191
-
EC Number 231-765-0
-
MDL number MFCD00011333
-
PubChem Substance ID 329757730

-
NACRES NA.21
Properties
| Related Categories | Chemical Synthesis, Oxidizing Agents, Peroxides, Synthetic Reagents |
| vapor pressure | 23.3 mmHg ( 30 °C) |
| concentration | 50 wt. % in H2O |
| mp | −40 °C |
| density | 1.197 g/mL at 20 °C |
| storage temp. | 2-8°C |
| SMILES string | OO |
| InChI | 1S/H2O2/c1-2/h1-2H |
| InChI key | MHAJPDPJQMAIIY-UHFFFAOYSA-N |
General description
The product is a 50% solution of hydrogen peroxide in water. Hydrogen peroxide is more commonly being used as a green oxidizing agent. It is a better alternative to other oxidants because it has high active oxygen content and the by-product is water.
Application
Hydrogen peroxide solution may be used as an oxidizing agent in the following reactions:
• Epoxidation of alkenes in the presence of homogeneous metal catalyst.[1]
• Oxidative halogenation in the presence of metal catalyst.[2]
• Oxidation of sulfides to sulfoxides and sulfones in the presence of polymer immobilised catalyst.[3]
Packaging
4 L in poly drum
500 mL in poly bottle
Other Notes
Contains proprietary inorganic tin-based stabilizer