Description
Glycerol
≥99.5%
Synonym(s):
1,2,3-Propanetriol, Glycerin
Linear Formula:
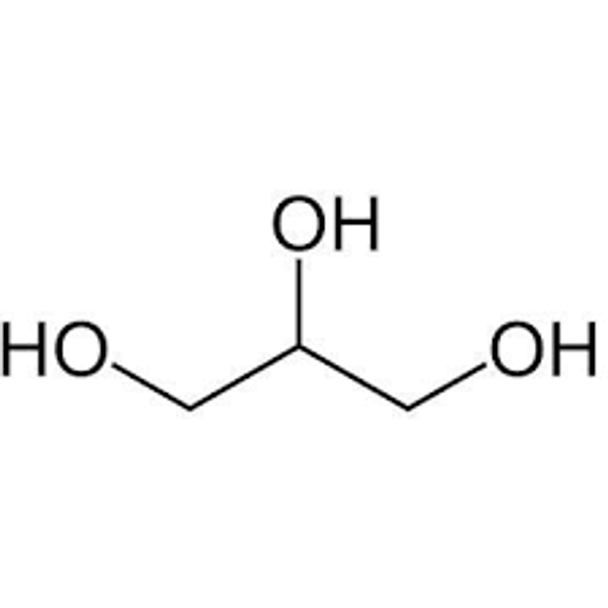
HOCH2CH(OH)CH2OH
CAS Number:
56-81-5
Molecular Weight:
92.09
Beilstein:
635685
EC Number:
200-289-5
MDL number:
MFCD00004722
PubChem Substance ID:
24895360
NACRES:
NA.21
PROPERTIES
vapor density
3.1 (vs air)
Quality Level
200
vapor pressure
<1 mmHg ( 20 °C)
Assay
≥99.5%
form
viscous liquid
autoignition temp.
698 °F
impurities
≤0.20% Water (Karl Fischer)
refractive index
n20/D 1.474 (lit.)
bp
182 °C/20 mmHg (lit.)
mp
20 °C (lit.)
density
1.25 g/mL (lit.)
cation traces
heavy metals (as Pb): ≤5 ppm
λ
1 cm path
UV absorption
λ: 205 nm Amax: 1.0
λ: 225 nm Amax: 0.40
λ: 280 nm Amax: 0.07
λ: 320 nm Amax: 0.02
λ: 340 nm Amax: 0.01
λ: 400 nm Amax: 0.01
suitability
suitable for component for culture media
SMILES string
OCC(O)CO
InChI
1S/C3H8O3/c4-1-3(6)2-5/h3-6H,1-2H2
Inchi Key
PEDCQBHIVMGVHV-UHFFFAOYSA-N
DESCRIPTION
General description
Glycerol is odourless, colorless, viscous in nature, and exists as a sweet tasting liquid. It can be derived naturally as well as from petrochemical feedstock. Glycerol has a wide variety of applications, and is one of the most valuable and versatile chemical substances in nature. It can be used as an emollient, solvent, sweetening agent, in pharmaceutical formulations, cosmetics, foodstuffs and toiletries. It is very stable and can be easily stored under normal temperature; also it is non-irritating and has no adverse impact to the environment.
Application
Glycerol has been used as a component of mounting medium for immunofluorescence.[1] It has also been used as additives in poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) based inks.[2]
Glycerol is used both in sample preparation and gel formation for polyacrylamide gel electrophoresis. Glycerol (5-10%) increases the density of a sample so that the sample will layer at the bottom of a gel′s sample well. Glycerol is also used to aid in casting gradient gels and as a protein stabilizer and storage buffer component.
Biochem/physiol Actions
Glycerol is hygroscopic in nature and is soluble in water owing to its three hydrophilic alcoholic hydroxyl groups. It can form both inter- and intramolecular hydrogen bonds, making it a very flexible molecule. The physiologic effect of glycerine is due to cell-mediated immunity, increased IgG production and increased histamine release.
SAFETY INFORMATION
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
390.2 °F - Pensky-Martens closed cup
Flash Point(C)
199 °C - Pensky-Martens closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves