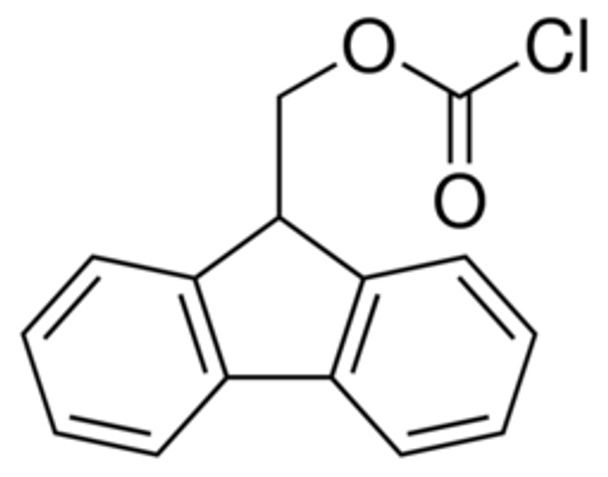
Description
Fmoc chloride 97%, 5G
Synonym: 9-Fluorenylmethoxycarbonyl chloride, 9-Fluorenylmethyl chloroformate, Fmoc-Cl
-
CAS Number 28920-43-6
-
Empirical Formula (Hill Notation) C15H11ClO2
-
Molecular Weight 258.70
-
Beilstein/REAXYS Number 2279177
-
EC Number 249-313-6
-
MDL number MFCD00001138
-
PubChem Substance ID 24849875

-
NACRES NA.22
Properties
| Related Categories | Amine Protection, Chemical Biology, Chemical Synthesis, Other Protecting and Derivatizing Reagents, Peptide Synthesis and Peptide Chemistry,
Protecting and Derivatizing Reagents, Protection and Derivatization, Synthetic Reagents
Less... |
| assay | 97% |
| application(s) | peptide synthesis: suitable |
| mp | 62-64 °C (lit.) |
| functional group | Fmoc |
| storage temp. | 2-8°C |
| SMILES string | ClC(=O)OCC1c2ccccc2-c3ccccc13 |
| InChI | 1S/C15H11ClO2/c16-15(17)18-9-14-12-7-3-1-5-10(12)11-6-2-4-8-13(11)14/h1-8,14H,9H2 |
| InChI key | IRXSLJNXXZKURP-UHFFFAOYSA-N |
Packaging
1, 5, 25 g in glass bottle
Application
Amino acid derivatizing agent for HPLC analysis.[1] N-protecting reagent for peptide[2] and oligonucleotide[3] syntheses.
Reagent for amino group protection recently used in the synthesis of a bicyclic proline analog.[4]
Reagent for derivatizing amino acids for HPLC amino acid analysis and for preparing N-Fmoc amino acids for solid-phase peptide synthesis.
Safety Information
Signal word
Danger
Hazard statements
H314
Precautionary statements
P260 - P280 - P301 + P330 + P331 - P303 + P361 + P353 - P304 + P340 + P310 - P305 + P351 + P338
Personal Protective Equipment
Eyeshields, Faceshields, Gloves, respirator cartridge type N100 (US), type P1 (EN143) respirator filter, type P3 (EN 143) respirator cartridges
RIDADR
UN 3261 8 / PGII
WGK Germany
WGK 3
RTECS
LQ6250000
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable