Description
Caftaric acid, ≥97.0%, 5MG
Synonym(s):
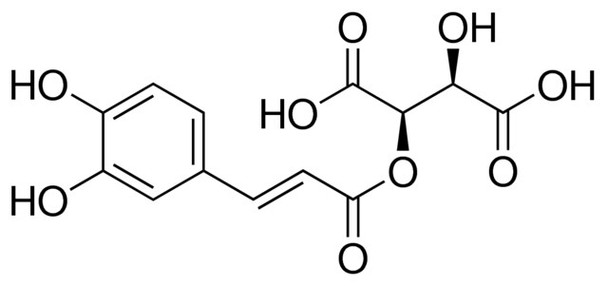
2-Caffeoyl-L-tartaric acid
Empirical Formula (Hill Notation):
C13H12O9
CAS Number:
67879-58-7
Molecular Weight:
312.23
MDL number:
MFCD02094177
PubChem Substance ID:
329750685
NACRES:
NA.25
Quality Level
100
assay
≥97.0%
form
powder
impurities
≤10% water
storage temp.
2-8°C
SMILES string
O[C@H]([C@@H](OC(=O)\C=C\c1ccc(O)c(O)c1)C(O)=O)C(O)=O
InChI
1S/C13H12O9/c14-7-3-1-6(5-8(7)15)2-4-9(16)22-11(13(20)21)10(17)12(18)19/h1-5,10-11,14-15,17H,(H,18,19)(H,20,21)/b4-2+/t10-,11-/m1/s1
InChI key
SWGKAHCIOQPKFW-JTNORFRNSA-N
Cafteric acid (CFA) is a polyphenolic compound belonging to the hydroxycinnamic acids (HCAs) subgroup. It is a derivative of caffeic acid. Cafteric acid is mainly sourced from Echinacea purpurea.
Application
Caftaric acid has been used:
- as a standard antioxidant to determine the antioxidant potential (AOP) of red wine using 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay
- as an antioxidant together with sulfur dioxide (SO2) to measure the antioxidant potential of white wines using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and Folin-Ciocalteu (FC) assays
- to evaluate the myelopoietic effect on bone marrow of rats treated with various Echinacea purpurea extracts
- to identify (poly)phenolic compounds in concord grape juice and their metabolites in human plasma and urine after juice consumption
Biochem/physiol Actions
Caftaric acid exerts antioxidant and anti-inflammatory effects against indomethacin-induced gastric ulcers in rats. It also displays antimutagenicity properties.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves