Description
Caffeine, Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(s):
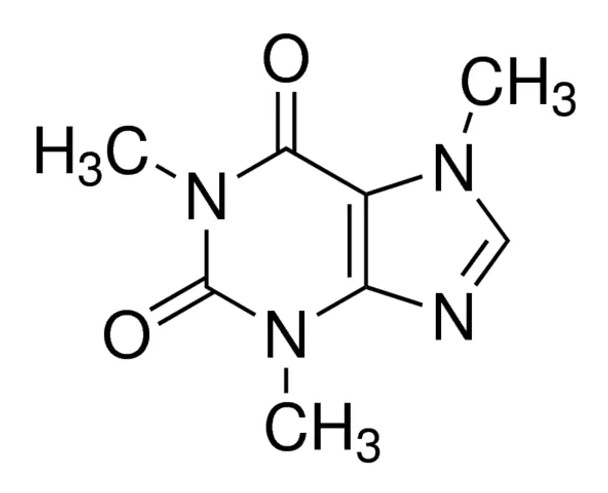
1,3,7-Trimethylxanthine
Empirical Formula (Hill Notation):
C8H10N4O2
CAS Number:
58-08-2
Molecular Weight:
194.19
Beilstein:
17705
MDL number:
MFCD00005758
PubChem Substance ID:
329823040
NACRES:
NA.24
grade
certified reference material
pharmaceutical secondary standard
Quality Level
300
Agency
traceable to BP 766
traceable to Ph. Eur. C0100000
traceable to USP 1085003
CofA
current certificate can be downloaded
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
mp
234-236.5 °C (lit.)
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-30°C
SMILES string
CN1C(=O)N(C)c2ncn(C)c2C1=O
InChI
1S/C8H10N4O2/c1-10-4-9-6-5(10)7(13)12(3)8(14)11(6)2/h4H,1-3H3
InChI key
RYYVLZVUVIJVGH-UHFFFAOYSA-N
Gene Information
human ... ADORA1(134) , ADORA2A(135) , ADORA2B(136) , ADORA3(140)
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to pharmacopeia primary standards. Caffeine is an N-methyl derivative of xanthine and is involved in gastric acid secretion, diuresis, and stimulation of the central nervous system.
Application
Caffeine may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations, blood plasma, food, drinks, and herbal products by chromatography techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Biochem/physiol Actions
A central nervous system stimulant believed to act through adenosine receptors and monoamine neurotransmitters. It is an adenosine receptor antagonist and adenosine 3′,5′-cyclic monophosphate (cAMP) phosphodiesterase inhibitor. Thus, levels of cAMP increase in cells following treatment with caffeine. It has been reported to affect cellular calcium levels, releasing calcium from intracellular stores. It overrides the cell cycle effects of various chemicals such as protease inhibitors, preventing apoptosis; and it has been shown to inhibit cellular DNA repair mechanisms.
Analysis Note
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
Other Notes
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Footnote
To see an example of a Certificate of Analysis for this material enter LRAB7780 in the slot below. This is an example certificate only and may not be the lot that you receive.
SAFETY INFORMATION
Pictograms
GHS07
Signal Word
Warning
Hazard Statements
H302
Precautionary Statements
P264 - P270 - P301 + P312 - P501
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 1