Description
Arachidonoyl dopamine ≥98% (HPLC), ethanol solution, 5MG
Synonym(s):
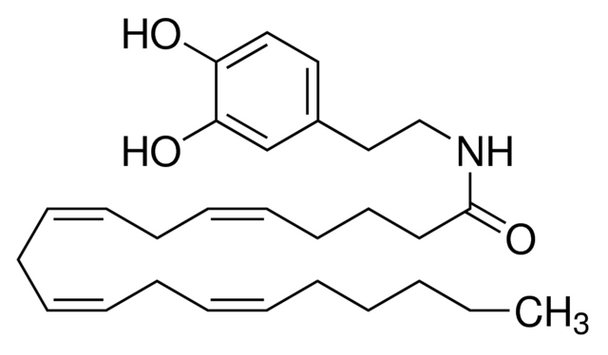
AA-DA, Arachidonyl dopamine, N-[2,3-(4-Dihydroxyphenyl)ethyl]-5Z,8Z,11Z,14Z-eicosatetraenamide, NADA
Empirical Formula (Hill Notation):
C28H41NO3
Molecular Weight:
439.63
MDL number:
MFCD03412031
PubChem Substance ID:
24891376
NACRES:
NA.77
Quality Level
200
assay
≥98% (HPLC)
form
ethanol solution
drug control
regulated under CDSA - not available from Sigma-Aldrich Canada
shipped in
wet ice
storage temp.
−20°C
SMILES string
CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCCc1ccc(O)c(O)c1
InChI
1S/C28H41NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-28(32)29-23-22-25-20-21-26(30)27(31)24-25/h6-7,9-10,12-13,15-16,20-21,24,30-31H,2-5,8,11,14,17-19,22-23H2,1H3,(H,29,32)/b7-6-,10-9-,13-12-,16-15-
InChI key
MVVPIAAVGAWJNQ-DOFZRALJSA-N
The endogenous cannabinoid system is comprised of two cannabinoid-receptor subtypes (CB1 and CB2 endocannabinoids (endogenous agonists of the receptors), and other supporting proteins. This system is involved in brain neuromodulation and has been reported to affect physiological processes such as appetite, pain, and movement, as well as others. Arachidonoyl Dopamine (AA-DA) is the amide of the neurotransmitter bioactive amine, dopamine, and the polyunsaturated fatty acid, arachidonic acid. AA-DA displays cannabimimetic activity in vitro and in vivo, has been shown to activate the CB1 receptor, and is a ligand for the endogenous VR1 vanilloid receptor.
Application
Used in studies of the endogenous cannabinoid system.
Biochem/physiol Actions
AA-DA competitively inhibits fatty acid amide hydrolase (IC50 = approx. 22 μM) from N18TG2 neuroblastoma cells and inhibits binding (Ki = 0.25 μM) of the selective cannabinoid receptor subtype 1 (CB1) ligand, [3H]SR141716A, to rat brain membrane. AA-DA also inhibits the anandamide membrane transporter in BL-2H3 basophilic leukemia and C6 glioma cells. AA-DA has at least a 40 fold greater selectivity for CB1 than CB2 receptors in rat brain membranes and has been shown to be more potent than anandamide as a CB1 agonist in undifferentiated N18TG2 neuroblastoma cells. AA-DA induces hypothermia and immobility, and decreases spontaneous activity and pain perception in mice and rats, which supports its action as a CB1 agonist in vivo. AA-DA has been shown to inhibit (IC50 = 0.25 μM) proliferation of human breast MCF-7 cancer cells.
Other Notes
Tandem Mass Spectrometry data independently generated by Scripps Center for Metabolomics is available to view or download in PDF. A8848.pdf Tested metabolites are featured on Scripps Center for Metabolomics METLIN Metabolite Database.
SAFETY INFORMATION
Pictograms
GHS02,GHS07
Signal Word
Danger
Hazard Statements
H225 - H319
Precautionary Statements
P210 - P305 + P351 + P338
RIDADR
UN1170 - class 3 - PG 2 - Ethanol, solution
WGK Germany
WGK 3
Flash Point(F)
57.2 °F - closed cup
Flash Point(C)
14.0 °C - closed cup