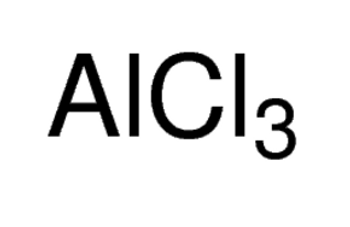Description
Aluminum chloride, ReagentPlus®, 99%, 100G
Linear Formula:
AlCl3
CAS Number:
7446-70-0
Molecular Weight:
133.34
EC Number:
231-208-1
MDL number:
MFCD00003422
eCl@ss:
38120507
PubChem Substance ID:
329752441
NACRES:
NA.21
PROPERTIES
vapor pressure
1 mmHg ( 100 °C)
Quality Level
200
product line
ReagentPlus®
Assay
99%
form
powder
reaction suitability
reagent type: catalyst
core: aluminum
pH
2.4 (20 °C, 100 g/L)
mp
190 °C (lit.)
SMILES string
Cl[Al](Cl)Cl
InChI
1S/Al.3ClH/h;3*1H/q+3;;;/p-3
Inchi Key
VSCWAEJMTAWNJL-UHFFFAOYSA-K
General description
Aluminum chloride is an alcohol-soluble lewis acid.† Aluminum chloride mixed with sodium borohydride, in diglyme in a molar ratio affords a mixture having strong reducing properties.† It was reported to promote the Friedel-Crafts acylation reaction between 4-tert-butylbenzoyl chloride and mesitylene.†
Application
Aluminum chloride was used for the following studies:
- Polymerization of benzene to afford p-polyphenyl.[1]
- As a catalyst for the Friedel-Crafts acylation of buckymetallocenes with the acid chlorides.[2]
- As a catalyst for the colorimetric microassay for the determination of lipid hydroperoxides.[3]
Features and Benefits
The vapor-phase co-reductions with other metal halides by hydrogen results in finely divided intermetallics with applications as structural materials or compounds with useful thermoelectric, magnetic, and oxidation-resistance properties. Aluminum nitride can be synthesized from the elemnts with AlCl3 added to help facilitate crystallization.
Legal Information
ReagentPlus is a registered trademark of Sigma-Aldrich Co. LLC
SAFETY INFORMATION
Pictograms

GHS05
Signal Word
Danger
Hazard Statements
H314
Precautionary Statements
P260 - P280 - P303 + P361 + P353 - P304 + P340 + P310 - P305 + P351 + P338 - P363
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Supplementary Hazards
EUH014 - EUH071
Storage Class Code
8B - Non-combustible, corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable