Description
Acetone
Laboratory Reagent, ≥99.5%
Linear Formula:
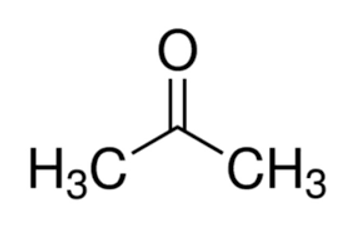
CH3COCH3
CAS Number:
67-64-1
Molecular Weight:
58.08
Beilstein:
635680
EC Number:
200-662-2
MDL number:
MFCD00008765
eCl@ss:
39021201
PubChem Substance ID:
329751600
NACRES:
NA.21
PROPERTIES
grade
Laboratory Reagent
Quality Level
100
vapor density
2 (vs air)
vapor pressure
184 mmHg ( 20 °C)
Assay
≥99.5%
form
liquid
expl. lim.
13.2 %
impurities
≤0.5% water
evapn. residue
≤0.002%
refractive index
n20/D 1.359 (lit.)
pH
5-6 (20 °C, 395 g/L)
bp
56 °C/760 mmHg (lit.)
mp
−94 °C (lit.)
density
0.791 g/mL at 25 °C (lit.)
format
neat
SMILES string
CC(C)=O
InChI
1S/C3H6O/c1-3(2)4/h1-2H3
InChI key
CSCPPACGZOOCGX-UHFFFAOYSA-N
DESCRIPTION
General description
Acetone is a polar organic solvent. It can undergo photocatalytic oxidation in the presence of mixed TiO2-rare earth oxides.[1]
Application
Acetone undergoes aldolization in the presence of Mg-Al layered double hydroxides (LDH) as catalysts and Cl- and/or CO32- as compensating anions to afford diacetone alcohol and mesityl oxide as the main products.[2] Its enantioselective Aldol condensation with various isatins in the presence of a dipeptide catalyst forms 1-alkyl 3-(2-oxopropyl)-3-hydroxyindolin-2-ones.[3] Aqueous solution of acetone may be used as a medium for the oxidation of alkynes to 1,2-diketones using potassium permanganate.[4]
Acetone′s luminesence intensity is dependent upon the solution components . The absorption of UV light by acetone, results in its photolysis and the production of radials .
SAFETY INFORMATION
Signal Word
Danger
Hazard Statements
H225 - H319 - H336
Precautionary Statements
P210 - P233 - P240 - P241 - P242 - P305 + P351 + P338
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Target Organs
Central nervous system
Supplementary Hazards
EUH066
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17.0 °C - closed cup