Description
Acetone
HPLC Plus, for HPLC, GC, and residue analysis, ≥99.9%
-
CAS Number 67-64-1
-
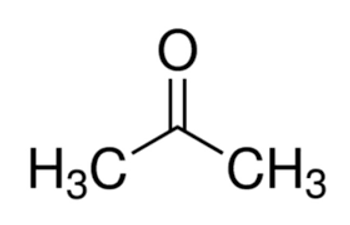
Linear Formula CH3COCH3
-
Molecular Weight 58.08
-
Beilstein/REAXYS Number 635680
-
EC Number 200-662-2
-
MDL number MFCD00008765
-
eCl@ss 39021201
-
PubChem Substance ID 329760025
Properties
| Related Categories | Acetone, Amber Glass Bottles, Analytical Reagents, Analytical/Chromatography, Chromatography Reagents & Solvents, |
| grade | HPLC Plus |
| for GC | |
| for HPLC | |
| for residue analysis | |
| vapor density | 2 (vs air) |
| vapor pressure | 184 mmHg ( 20 °C) |
| InChI Key | CSCPPACGZOOCGX-UHFFFAOYSA-N |
| assay | ≥99.9% |
| form | liquid |
| expl. lim. | 13.2 % |
| impurities | ≤0.5% water |
| ≤1.0 ppb Fluorescence (quinine) at 365 nm | |
| evapn. residue | ≤0.0003% |
| halogenated residue | <10 ng/L (as heptachlor epoxide) |
| refractive index | n20/D 1.359 (lit.) |
| bp | 56 °C/760 mmHg (lit.) |
| mp | −94 °C (lit.) |
| solubility | water: miscible |
| density | 0.791 g/mL at 25 °C (lit.) |
| λ | H2O reference |
| UV absorption | λ: 330 nm Amax: 1.0 |
| λ: 340 nm Amax: 0.06 | |
| λ: 350 nm Amax: 0.01 | |
| λ: 375-400 nm Amax: 0.005 | |
| Featured Industry | Food and Beverages |
General description
Acetone is a ketone. Mixtures of TiO2 (P25) with rare earth oxides (La2O3 or Y2O3) calcined at 700°C or 650°C has been reported to efficiently photocatalyze oxidation of acetone.[1] Its aldol condensation in vapor-phase has been investigated over MgO promoted with alkali (Li, Na, K and Cs) or alkaline earth (Ca, Sr and Ba) metal ions.[2] It undergoes Aldol condensation in the presence of Mg-Al layered double hydroxides (LDH) as catalysts and Cl- and/or CO32- as compensating anions to afford diacetone alcohol and mesityl oxide.[3] Enantioselective Aldol condensation of acetone with various isatins in the presence of dipeptides (catalyst) has been described.[4]
Application
Acetone may be used in the following studies:
• NMR spectroscopic evaluation of glucose metabolism in biological samples (blood).[5]
• Preparation of 13wt% amorphous poly (lactic acid) (PLA) solution, which was required for the preparation of electrospun PLA membranes.[6]
• As a precursor for the synthesis of methyl isobutyl ketone (MIBK) in the presence of sulfonated graphene oxide-Pd/cordierite catalyst.[7]
• Synthesis of (4-hydroxymethyl-2,2-dimethyl-1,3-dioxolane), a solketal from glycerol using supercritical fluids (SCF) technology.[8]
It may be used in the synthesis of the following acetone hydrazones:
• 1-isopropylidene-2-methylhydrazine[9]
• 1-isopropylidene-2-hydroxyethylhydrazine[9]
• 1-isopropylidene-2-formylhydrazine[9]
Acetone′s luminesence intensity is dependent upon the solution components . The absorption of UV light by acetone, results in its photolysis and the production of radials .
Suitable for HPLC, spectrophotometry, environmental testing
Packaging
1, 6×1, 4, 4×4 L in glass bottle