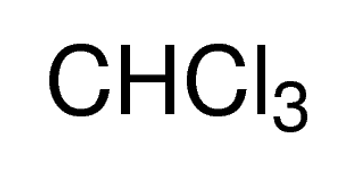Description
1-Methyl-2-pyrrolidinone
biotech. grade, ≥99.7%
PROPERTIES
InChI key
SECXISVLQFMRJM-UHFFFAOYSA-N
grade
biotech. grade
Quality Level
100
vapor density
3.4 (vs air)
vapor pressure
0.29 mmHg ( 20 °C)
0.99 mmHg ( 40 °C)
assay
≥99.7%
form
liquid
autoignition temp.
518 °F
expl. lim.
9.5 %
impurities
≤0.005% water
≤0.01% free amines (CH3NH2)
color
APHA: ≤20
refractive index
n20/D 1.47 (lit.)
pH
7.7-10.0 (20 °C, 100 g/L)
bp
202 °C (lit.)
81-82 °C/10 mmHg (lit.)
mp
−24 °C (lit.)
solubility
acetone: miscible(lit.)
alcohol: miscible(lit.)
chloroform: miscible(lit.)
ethyl acetate: miscible(lit.)
water: miscible(lit.)
density
1.028 g/mL at 25 °C (lit.)
λ
H2O reference
UV absorption
λ: 285 nm Amax: 1.00
λ: 300 nm Amax: 0.50
λ: 325 nm Amax: 0.10
λ: 350-400 nm Amax: 0.01
application(s)
peptide synthesis
SMILES string
CN1CCCC1=O
InChI
1S/C5H9NO/c1-6-4-2-3-5(6)7/h2-4H2,1H3
General description
SAFETY INFORMATION
pictograms
signalword
Danger
hcodes
pcodes
Hazard Classifications
Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 1
flash_point_f
195.8 °F - Pensky-Martens closed cup
flash_point_c
91 °C - Pensky-Martens closed cup